Clazosentan
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
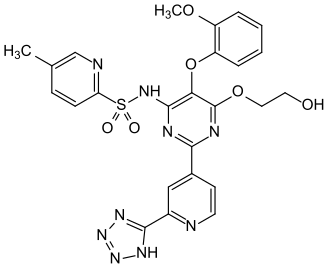
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Clazosentan | |||||||||||||||
| other names |
5-methyl-pyridin-2-sulfonic acid {6- (2-hydroxy-ethoxy) -5- (2-methoxyphenoxy) -2- [2- (1 H -tetrazol-5-yl) -pyridin-4- yl] pyrimidin-4-yl} amide |
|||||||||||||||
| Molecular formula | C 25 H 23 N 9 O 6 S | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 577.57 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Clazosentan is an experimental drug from the group of endothelin receptor antagonists .
Clazosentan is being developed by the Swiss pharmaceutical company Actelion . The disodium salt is used medicinally .
Mechanism of action
The endothelin -1 receptor is one of the most powerful vasoconstrictors known . Clazosentan acts as an antagonist on this receptor . After subarachnoid hemorrhage, irritation of the blood vessels can lead to vasospasm and, associated with this, to an insufficient supply of oxygen to the brain tissue . One possible consequence can be an ischemic stroke . Clazosentan counteracts this vasoconstriction .
The plasma half-life is 6–10 min .
Studies
Clazosentan was tested from 2007 in phase III of the clinical studies CONSCIOUS-2 ( Clazosentan to Overcome Neurological Ischemia and Infarct Occurring after Subarachnoid hemorrage ) for the treatment of vasospasm after subarachnoid hemorrhage ( narrowing of blood vessels after severe cerebral hemorrhage). No significant effects on vasospasm-related morbidity or overall mortality could be found, but undesirable effects such as pulmonary complications, anemias and hypotension increased. Another study (CONSCIOUS-3) did not produce the desired results either.
Clazosentan was to be marketed under the name Pivlaz .
literature
- R. Loch Macdonald, Randall T. Higashida, Emanuela Keller, Stephan A. Mayer, Andy Molyneux, Andreas Raabe, Peter Vajkoczy, Isabel Wanke, Doris Bach, Aline Frey, Angelina Marr, Sébastien Roux, Neal Kassell: Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomized, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). In: The Lancet. Neurology . tape 10 , no. 7 , 2011, p. 618-625 , doi : 10.1016 / S1474-4422 (11) 70108-9 , PMID 21640651 .
- Paul LM Van Giersbergen, J. Dingermanse: Tolerability, pharmacokinetics, and pharmacodynamics of clazosentan, a parenteral endothelin receptor antagonist . In: European Journal of Clinical Pharmacology . tape 63 , no. 2 , February 2007, p. 151–158 , doi : 10.1007 / s00228-006-0117-z , PMID 16636870 .
- Paul LM Van Giersbergen, J. Dingermanse, KA Gunawardena: Influence of ethnic origin and sex on the pharmacokinetics of clazosentan . In: Journal of Clinical Pharmacology . tape 47 , no. November 11 , 2007, pp. 1374-1380 , doi : 10.1177 / 0091270007307337 , PMID 17906281 ( sagepub.com ).
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ DH Rhoney, K. Morbitzer, J. Hatton-Kolpek: Pharmacotherapy for Cerebral vasospasm prophylaxis and treatment in Subarachnoid Hemorrhage . In: Neuropharmacotherapy in Critical Illness, Gretchen Brophy (Ed.), Rutgers University Press, 2017; P. 144.