Collidine
In chemistry , the collidines (sometimes also collidines ) or trimethylpyridines form a group of organic compounds that belong to the heterocycles (more precisely: heteroaromatic compounds ). They consist of a pyridine ring that is substituted with three methyl groups. Their different arrangement results in six constitutional isomers with the empirical formula C 8 H 11 N. The 2,4,6-collidine is the best-known isomer.
properties
The collidines are colorless liquids with a pyridine-like odor. They are soluble in water and ethanol .
| Collidine | |||||||||||||
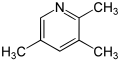
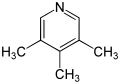
| Surname | 2,3,4-collidine | 2,3,5-collidine | 2,3,6-collidine | 2,4,5-collidine | 2,4,6-collidine | 3,4,5-collidine | |||||||
| other names | 2,3,4-trimethylpyridine |
2,3,5-trimethylpyridine |
2,3,6-trimethylpyridine |
2,4,5-trimethylpyridine |
2,4,6-trimethylpyridine, sym- collidine |
3,4,5-trimethylpyridine |
|||||||
| Structural formula |

|
|

|

|

|
|
|||||||
| CAS number | 2233-29-6 | 695-98-7 | 1462-84-6 | 1122-39-0 | 108-75-8 | 20579-43-5 | |||||||
| PubChem | 16691 | 12759 | 15100 | 14280 | 7953 | 88601 | |||||||
| Molecular formula | C 8 H 11 N | ||||||||||||
| Molar mass | 121.18 g mol −1 | ||||||||||||
| Physical state | liquid | ||||||||||||
| Brief description | colorless liquids with a pyridine-like odor | ||||||||||||
| Melting point | −43 ° C | ||||||||||||
| boiling point | 192-193 ° C | 184 ° C | 176-178 ° C | 165-168 ° C | 171-172 ° C | ||||||||
|
pK s value (25 ° C) (of the conjugate acid BH + ) |
7.43 | ||||||||||||
| solubility | 35 g l −1 | ||||||||||||
|
GHS labeling |
|
|
|
|
|
|
|||||||
| H and P phrases | see above | 315-319-335 | see above | see above | 226-302-311-315-319-332-335 | see above | |||||||
| see above | no EUH phrases | see above | see above | no EUH phrases | see above | ||||||||
| see above | 261-305 + 351 + 338 | see above | see above | 261-280-305 + 351 + 338-312 | see above | ||||||||
See also
Individual evidence
- ↑ Entry on Kollidin. In: Römpp Online . Georg Thieme Verlag, accessed on November 25, 2014.
- ↑ a b c d Entry on 2,4,6-trimethylpyridine in the GESTIS substance database of the IFA , accessed on April 20, 2017(JavaScript required) .
- ↑ a b c d e Zvi Rappoport: CRC Handbook of Tables for Organic Compound Identification , Third Edition, CRC Press, Boca Raton, Florida, 1984, ISBN 0-8493-0303-6 .
- ↑ Data sheet 2,3,5-Collidine from Sigma-Aldrich , accessed on April 20, 2017 ( PDF ).
Web links
Commons : Collidine - collection of pictures, videos and audio files


