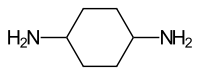
Cyclohexane-1,4-diamine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Simplified structural formula without specifying the relative stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyclohexane-1,4-diamine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 14 N 2 | |||||||||||||||
| Brief description |
brownish crystal pulp with an amine-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 114.19 g mol −1 | |||||||||||||||
| Physical state |
liquid or solid, depending on the composition |
|||||||||||||||
| density |
approx. 0.9 g cm −3 |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Cyclohexane-1,4-diamine is a chemical compound from the group of aliphatic amines .
Isomers
Cyclohexane-1,4-diamine occurs in two isomeric forms :
- cis -cyclohexane-1,4-diamine and
- trans -cyclohexane-1,4-diamine.
Extraction and presentation
Cyclohexane-1,4-diamine can be obtained by amination of cyclohexane-1,4-diol in supercritical ammonia at high pressure.
properties
Cyclohexane-1,4-diamine, as a mixture of isomers, is a brownish crystal paste with an amine-like odor, which is soluble in water. Its physical state (liquid or solid) depends on the composition. The cis isomer is liquid, the mixture of isomers is solid. Its aqueous solution has an alkaline reaction.
use
trans -cyclohexane-1,4-diamine is used to make fully aliphatic polyimides . It is also used as a structure-directing agent in the synthesis of novel two-dimensionally layered zinc phosphates.
Individual evidence
- ↑ a b c d e f g h Entry on cyclohexane-1,4-diamine in the GESTIS substance database of the IFA , accessed on December 27, 2016(JavaScript required) .
- ↑ a b c d data sheet trans-1,4-diaminocyclohexane, ≥98.0% (GC) from Sigma-Aldrich , accessed on December 27, 2016 ( PDF ).
- ↑ External identifiers or database links for cis-1,4-cyclohexanediamine : CAS number: 15827-56-2, EC number: 640-359-6, ECHA InfoCard: 100.167.991 , Wikidata : Q64027380 .
- ↑ External identifiers or database links for trans-1,4-cyclohexanediamine : CAS number: 2615-25-0, EC number: 640-403-4, ECHA InfoCard: 100.168.150 , Wikidata : Q64027383 .
- ↑ A. Fischer, T. Mallat, A. Baiker: Synthesis of 1,4-Diaminocyclohexane in Supercritical Ammonia. In: Journal of Catalysis. 182, 1999, p. 289, doi : 10.1006 / jcat.1999.2410 .

