Polyimides
Polyimides ( abbreviation PI ) are plastics whose most important structural feature is the imide group . These include polysuccinimide (PSI), polybismaleimide (PBMI), polyimide sulfone (PISO) and polymethacrylimide (PMI).
Polyimides that contain further structural elements such as ester groups, amide groups, etc., form their own groups of substances such as polyetherimides (PEI) and polyamideimides (PAI).
Manufacturing
There are several methods of making polyimides including:
- by polycondensation between tetracarboxylic dianhydrides and diamines (the most commonly used method).
- by the reaction between dianhydrides and diisocyanates .
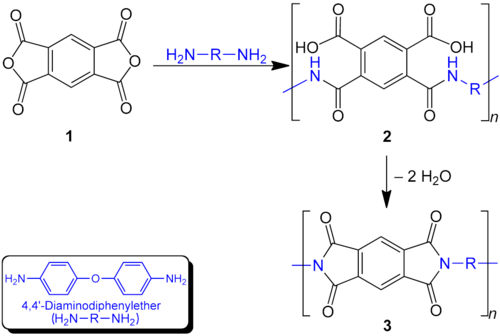
Usual dianhydrides are u. a. Pyromellitic dianhydride , benzoquinone tetracarboxylic dianhydride and naphthalenetetracarboxylic dianhydride . Common diamines are 4,4'-diaminodiphenyl ether ("DAPE"), 4,4'-diaminodiphenyl sulfone ( Dapsone ), meta- phenylenediamine ("MDA") and 3,3-diaminodiphenylmethane.
The polycondensation between tetracarboxylic acid dianhydrides and diamines is carried out in a 2-stage process in which a polyamidocarboxylic acid ( 2 ) is formed first and this is only condensed to the polyimide ( 3 ) in a second step . The reason for this is that most commercial products contain aromatic building blocks in the polymer chain and such products can no longer be processed in liquid form after condensation to the imide (because of their insolubility and extremely high or missing melting points). The following scheme shows the procedure:
The anhydride ( 1 ) is reacted with the diamine in an anhydrous, polar solvent such as N- methyl-2-pyrrolidone (NMP) or dimethylformamide (DMF) to form the polyamidocarboxylic acid ( 2 ). The solution obtained in this way can be poured or applied as a paint. After evaporation of the solvent and application of high temperatures (enamelling), the conversion to the finished polyimide ( 3 ) takes place with elimination of water . A typical application is enamelled wire insulation . Since the polyamidocarboxylic acids are quite corrosive and the process is often time-consuming for the user, an attempt is made to give preference to the step of imide formation in the liquid products as far as possible. Photosensitive polyimides for printed circuits etc. can be obtained by esterification of the acid groups in (B) with methacrylic acid via glycols . These can be fixed by exposure, the unexposed areas dissolved again and then the fixed material thermally converted to polyimide.
Properties and uses

Purely aromatic polyimides are often not meltable and chemically very resistant (also to many solvents). Polyimide dissolved in the solvents DMF , DMAc or NMP is suitable for use as a coating agent . Polyimides are used in electrical engineering / electronics because of their heat resistance, low outgassing, radiation resistance and insulating properties in the form of light brownish, semi-transparent films . High continuous use temperatures of up to 230 ° C and briefly up to 400 ° C are possible. Property profiles can be found in dealer and manufacturer information.
Polyimides are used for particularly thin and yet very stable lacquer insulation of electrical lines, u. a. to save weight in aircraft technology. Due to their small thickness compared to the conductor, such insulation is sensitive to mechanical loads regardless of the material (see also arc tracking ).
For the generation of X-rays , polyimides are used as versatile, stable and inexpensive materials for X-ray windows. The prerequisites for this are their thermal stability and high transmittance for X-ray light. If a high level of absorption and reflection is also required for light in the visible range, beryllium is usually used.
Polyimide films are also used in reverse osmosis as selectively permeable membranes - for example in dialysis or in seawater desalination.
Construction parts and semi-finished products made of polyimide are manufactured from polyimide powder using sintering techniques such as hot compression molding , direct forming or isostatic pressing . Due to the necessary high mechanical strength even at high temperatures, bushings, bearings, guides and sealing rings made of PI are used in thermally demanding applications. The tribological properties can be specifically improved in particular by using solid lubricants such as graphite, molybdenum disulphide or PTFE.
Polyimide fibers, which belong to the group of polyheterocyclic fibers, do not have any outstanding mechanical-physical properties, but with an LOI value of 38 they are characterized by infusibility, high thermal stability and flame retardancy. Continuous use temperatures of 260 ° C and peak temperatures of up to 300 ° C can be achieved in practice. This type of fiber is resistant to the usual organic solvents and acids. There are reservations for use in alkaline environments. PI fibers are particularly suitable as microfibers (fineness 0.6 and 1.0 dtex ) in needle punched nonwovens for filters in hot gas filtration . Fine dusts are separated from the exhaust gas from coal-fired power plants, waste incineration plants or cement factories at exhaust gas temperatures between 160 ° C and 260 ° C, for which the multilobal fiber cross-section is also important. Applications for flame-retardant protective clothing, braided sealing packs and synthetic paper webs for pressboards are also known, since crimped staple fibers, filament yarns and fibrids can be produced from the PI fiber material.
For the foldable smartphone Galaxy X1 from Samsung Electronics planned for 2018 , polyimide film from the South Korean company Kolon Industries is to be used. Kolon Industries was the first company in the world to produce colorless polyimide film.
Trade names
A well-known trade name for polyimide films, which are mainly used for further use in the electrical industry, is Kapton from DuPont . According to EN 62368-1 , the electric breakdown field strength is 303 kV / mm and is therefore the material with the highest breakdown field strength in Table 21.
Other well-known brands are:
- Vespel from DuPont
- Apical from Kaneka Americas Holding Inc.
- Kinel from Vyncolit NV
- Message from Saint Gobain
- P84 from Evonik Industries
- Upilex from Ube Industries Ltd.
literature
- Varun Ratta: POLYIMIDES: Chemistry & structure-property relationships - literature review (Chapter 1).
Individual evidence
- ^ Zvi Rappoport: The Chemistry of Anilines. John Wiley & Sons, 2007, ISBN 978-0-470-87172-0 , p. 773 ( limited preview in Google book search).
- ^ Walter W. Wright and Michael Hallden-Abberton "Polyimides" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi : 10.1002 / 14356007.a21_253
- ↑ ABC of lacquer and synthetic resin insulation for electrical engineering . Edited by BASF Colors + Fibers AG / Beck Elektro-Isolersysteme, 1974, 2nd edition.
- ↑ P. Mühlenbrock: Application of enamelled wires (PDF; 276 kB), 11th conference on electrical insulation systems 2004.
- ↑ Patent DE69705048 : Polyimide mixtures. Filed August 19, 1997 , published November 15, 2001 , applicant: DuPont , inventor: Sawyer Bloom.
- ↑ Patent DE69702867 : Process for the production of injection moldable polyimide resin compositions and shaped bodies. Filed November 21, 1997 , published January 18, 2001 , applicant: DuPont, inventor: Mureo Kaku.
- ↑ Crystec Technology Trading GmbH: Polyimide hardening, photosensitive and non-photosensitive polyimide .
- ↑ Leiton: Flexible printed circuit boards .
- ↑ Quick-ohm: Kapton insulating film (polyimide) .
- ↑ Müller-Ahlhorn: Kapton ( Memento of the original from February 9, 2010 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Multi-CB: Flexible circuit boards: standard layer structure polyimide .
- ↑ Walter Loy: Chemical fibers for technical textile products. 2nd, fundamental revised and expanded edition. Deutscher Fachverlag, Frankfurt am Main 2008, ISBN 978-3-86641-197-5 , p. 109.
- ↑ Michael Keller: Galaxy X1: Release probably not until the beginning of 2018. In: curved.de. January 12, 2017. Retrieved January 23, 2017 .
- ↑ EN 62368-1: 2014 + AC: 2015: Table 21 - Electric field strength E P for some commonly used materials