Dapsone
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
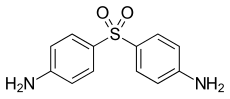
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Dapsone | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 12 H 12 N 2 O 2 S | |||||||||||||||||||||
| Brief description |
white to yellowish white crystalline powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 248.31 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
172-175 ° C |
|||||||||||||||||||||
| solubility |
very heavy in water (0.38 g l −1 at 37 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Dapsone ( 4,4′-diaminodiphenyl sulfone ) is an antibiotic and anti-inflammatory drug. It is used to treat and prevent infectious diseases such as leprosy , malaria , actinomycete infections and Pneumocystis jirovecii - pneumonia in HIV patients. It is also used for the treatment of chronic inflammatory autoimmune diseases in which tissue infiltration by neutrophilic and eosinophilic inflammatory cells is observed, such as Duhring's dermatitis herpetiformis , linear IgA dermatosis , subcorneal pustular dermatosis , the erythema elevatum diutinum and other autoimmune blistering erythema elevatum diutinum . The D - N , N ′ digalactoside is also used.
Dapsone was first synthesized in Germany in 1908 by E. Fromm and patented by IG Farben in 1934 . It is marketed as a chemotherapeutic agent (against dermatoses and leprosy) by the Riemser company (trade name: Dapson-Fatol ® ). Since dapsone is listed in Table 2 of Regulation (EU) No. 37/2010 on pharmacologically active substances and their classification with regard to the maximum residue levels in food of animal origin , its use in food-producing animals is generally prohibited in the European Union.
The antibiotic mechanism of action is explained by the antimetabolic inhibition of the folic acid synthesis of the mycobacterium. Due to the strong development of resistance, Dapsone is preferably used in combination with other preparations, e.g. B. with rifampicin or clofazimin .
The side effects of Dapsone include dose-dependent hemolysis and methemoglobinemia , especially in the case of glucose-6-phosphate dehydrogenase deficiency .
Technically, Dapsone is used as a hardener for epoxy resins (for hot-curing, temperature-resistant systems) and for the production of polyimides .
Dapsone hypersensitivity syndrome
When using dapsone, 0.5–3.6% of patients develop a hypersensitivity reaction after 4–6 weeks after the start of therapy , which was first described in 1949 in leprosy patients in Nigeria and first named in 1951 as dapsone hypersensitivity syndrome. This is a serious idiosyncratic side effect with fever, skin rash and involvement of other organ systems, primarily the liver and the blood-forming system, up to and including failure. The mortality is 9.9%.
In a Chinese genome-wide association study , the HLA-B * 13: 01 allele of the human leukocyte antigen system was described as a genetic risk factor for hypersensitivity syndrome, which is associated with an odds ratio of 20.53. Since this allele is found in 2–20% in China, in 1.5% in Japan, in 1–12% in India and in 2–4% in Southeast Asia, but rarely in Europe or Africa, the development of a genetic test is recommended to test affected populations for the HLA-B * 13: 01 allele prior to initiating treatment with dapsone.
Risk assessment
Dapsone was included by the EU in 2014 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation in the Community's ongoing action plan ( CoRAP ). The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reason for the inclusion of Dapsone was concerns as a potential endocrine disruptor . The reassessment took place from 2016 and was carried out by Germany . A final report was then published.
Individual evidence
- ^ Entry on Dapsone. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b c d Entry on Dapsone in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Entry on Dapsone in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry on Dapsone in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on January 13, 2020.
- ↑ a b F.-R. Zhang, H. Liu et al. a .: HLA-B * 13: 01 and the Dapsone Hypersensitivity Syndrome. In: New England Journal of Medicine. 369, 2013, pp. 1620-1628, doi : 10.1056 / NEJMoa1213096 .
- ↑ Red List Online, 08/2009.
- ^ Mutschler drug effects, 8th edition, WVG Stuttgart.
- ↑ Medical microbiology and infectious diseases. 2nd edition, Urban Fischer Verlag.
- ^ Zvi Rappoport: The Chemistry of Anilines. John Wiley & Sons, 2007, ISBN 978-0-470-87172-0 , p. 773 ( limited preview in Google book search).
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Dapsone , accessed on March 26, 2019.