Polysuccinimide
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
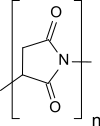
|
|||||||
| Structural element without information on stereochemistry | |||||||
| General | |||||||
| Surname | Polysuccinimide | ||||||
| other names |
|
||||||
| CAS number | 39444-67-2 | ||||||
| Monomer | Succinimide | ||||||
| Molecular formula of the repeating unit | C 4 H 3 NO 2 | ||||||
| Molar mass of the repeating unit | 97.07 g mol −1 | ||||||
| Type of polymer |
Polycondensation product |
||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| density |
0.55 g cm −3 |
||||||
| solubility |
|
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Polysuccinimide (PSI), also known as polyanhydroaspartic acid or polyaspartimide, is formed during the thermal polycondensation of aspartic acid and is the simplest polyimide . PSI is insoluble in water, but soluble in some aprotic dipolar solvents. Its reactive nature makes polysuccinimide a versatile starting material for functional polymers made from renewable raw materials.
The name is derived from that of the salts of succinic acid , the structurally related succinates .
presentation
The representation of PSI was reported by Hugo Schiff as early as 1897. When dry aspartic acid was heated for about 20 hours in a liquid bath at 190 ° C to 200 ° C, colorless products were obtained. Above 200 ° C there is a slight yellow coloration, the yield is almost quantitative.
In the Schiff experiments, oligomers and low molecular weight polymers were formed in a solid reaction through polycondensation with elimination of water, as generally in the absence of strong acids, which suppress the thermal decomposition of free amino end groups and thus chain termination reactions. The formation of the polyimide PSI can be followed using the intense absorption band in the infrared spectrum at 1714 cm −1 . In addition to the relatively low degree of polymerization, many process variants described in the patent literature often produce branched and yellow to brown-colored products.
In more recent work, the goal of increasing the molar mass and a linear chain structure while avoiding decomposition reactions was in the foreground. With a simple "oven process", in which a mixture or paste of crystalline aspartic acid and concentrated phosphoric acid or polyphosphoric acid is heated in a thin layer for 2 hours to 4 hours at 200 ° C, PSI falls with molar masses in the range of 30,000 g / mol and cream-white color. Carrying out the polycondensation in several steps (precondensation, comminution of the precondensate, postcondensation), with other dehydrating substances (e.g. zeolites , triphenyl phosphite ) or in the presence of solvents (e.g. propylene carbonate ) yields higher molecular weight products with molar masses in the range from 10,000 g / mol to 200,000 g / mol. In the patent literature, however, the polymer morphology, in particular the degree of branching, is not discussed.
A more recent patent describes the simple preparation of high molecular weight, practically colorless and linear, unbranched PSI by first dissolving the crystalline and practically water-insoluble aspartic acid, which is present as a zwitterion, with an aqueous, volatile acid, preferably hydrochloric acid, and adding the condensing agent phosphoric acid. The resulting homogeneous solution is evaporated at 120 ° C. and the glass-like mass obtained is then polycondensed at 180 ° C. to 200 ° C. for at least one hour. The phosphoric acid is washed out and the dried PSI is converted by mild alkaline hydrolysis into water-soluble polyaspartic acid, the molar mass of which can be determined by gel permeation chromatography . The process delivers reproducible PSI with molar masses over 100,000 g / mol.
Synthesis routes for PSI based on maleic acid monoammonium salt, maleic anhydride and ammonia or the intermediate maleic acid monoamide only achieve low molar masses of a few 1,000 g / mol and colored products, as do "green" process variants in supercritical carbon dioxide and avoiding Mineral acids as catalysts.
Because of the lower costs of the starting materials maleic anhydride and ammonia, which are produced from fossil raw materials, no L-aspartic acid is used in the manufacture of the commercial product Baypure® PSI.
properties
During thermal polycondensation, polysuccinimide is obtained as an odorless, non-hygroscopic, creamy white to brown powder, which is soluble in aprotic dipolar solvents such as dimethylformamide , dimethylacetamide , dimethyl sulfoxide , N -methylpyrrolidone , triethylene glycol or mesitylene / sulfolane mixture. PSI hydrolyzes very slowly in water. In dilute alkaline media (e.g. 1N sodium hydroxide solution ) hydrolysis occurs in the α- and β-position of the succinimide (2,5-pyrrolidinedione) ring structures and racemization at the chiral center of aspartic acid to the readily water-soluble sodium salt of poly (α, β) -DL-aspartic acid. About 30% of the α-shape and about 70% of the β-shape are formed in a random arrangement along the polymer chain.
At higher alkali concentrations or longer exposure times, the amide linkages in the polymer chain are also attacked with a reduction in the molar mass. The presence of amide bonds makes the polyaspartic acid obtained from hydrolysis even from highly crosslinked PSI relatively biodegradable (approx. 70% in waste water).
use
The polysuccinimide developed by Bayer AG and marketed by Lanxess AG under the brand name Baypure® DSP with a weight average molecular weight MW of 4.400 g / mol is already partially hydrolyzed at slightly higher pH values and thus swellable in a highly crosslinked form or in a linear manner Form water soluble. The copoly- (succinimide-aspartic acid) and especially polyaspartic acid (trade name Baypure® DS 100) produced by partial hydrolysis is suitable as a long-lasting inhibitor against limescale deposits in water treatment and in applications in the oil and mining industry, and as a setting retarder for cement in the Construction industry. In the patent literature PSI mentions applications as chelating agents, inhibitors against scale formation, dispersants and humectants, as well as fertilizer additives.
When the pyrrolidinedione ring structures in PSI are opened in the sense of aminolysis with ammonia water, poly (α, β) - DL -asparagine is formed, with hydrazine poly (α, β) -DL-aspartylhydrazide (PAHy) and with functional amines, e.g. . B. Ethanolamine poly (α, β) -DL-2-hydroxyethylaspartate (PHEA), which is characterized as a plasma expander with good biocompatibility and degradability, high water solubility at low manufacturing costs and more intensive as a potential drug carrier in medical applications was investigated.
Cross-linked poly (α, β) -DL-aspartic acid sodium salt as the commercially most interesting PSI derivative has been tested in extensive investigations for its suitability as a biodegradable superabsorbent in comparison to the non-biodegradable standard cross-linked sodium polyacrylate .
The results obtained have not yet led to the use of crosslinked polyaspartic acid in large-volume applications for superabsorbents (e.g. baby diapers ).
Individual evidence
- ↑ Baypure® Lanxess AG, DSP, Technical Information
- ↑ a b c Baypure® General Product Information (PDF) Lanxess AG
- ↑ E. Jalalvandi, A. Shavandi: Polysuccinimide and its derivatives: Degradable and water soluble polymers (review) . In: Eur. Polym. J. Band 109 , 2018, p. 43–54 , doi : 10.1016 / j.eurpolymj.2018.08.056 .
- ↑ T. Klein, R.-J. Moritz, R. Graupner: Ullmann's Polymers and Plastics, Products and Processes, Volume 1, Part 2: Organic Polymers, Polyaspartates and Polysuccinimide . Wiley-VCH, Weinheim 2016, ISBN 978-3-527-33823-8 , pp. 742-743 .
- ↑ M. Tomida, T. Nakato, M. Kuramochi, M. Shibata, S. Matsunami, T. Kakuchi: Novel method of synthesizig poly (succinimide) and its copolymeric derivatives by acid-catalysed polycondensation of L-aspartic acid . In: polymer . tape 37 , no. 16 , 1996, pp. 4435-4437 , doi : 10.1016 / 0032-3861 (96) 00267-4 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Hugo Schiff: About polyaspartic acids . In: Reports of the German Chemical Society . tape 30 , no. 3 , September 1, 1897, p. 2449-2459 , doi : 10.1002 / cber.18970300316 .
- ^ A b Kenneth Doll, Randal Shogren, Ronald Holser, J. Willett, Graham Swift: Polymerization of L-Aspartic Acid to Polysuccinimide and Copoly (Succinimide-Aspartate) in Supercritical Carbon Dioxide . In: Letters in Organic Chemistry . tape 2 , no. 8 , December 1, 2005, p. 687-689 , doi : 10.2174 / 157017805774717553 .
- ↑ Thomas Klein, Ralf-Johann Moritz, René Graupner: Polyaspartates and Polysuccinimide . In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH Verlag GmbH & Co. KGaA, 2008, ISBN 978-3-527-30673-2 , doi : 10.1002 / 14356007.l21_l01 .
- ^ A b Paolo Neri, Guido Antoni, Franco Benvenuti, Francesco Cocola, Guido Gazzei: Synthesis of α, β- poly [(2-hydroxyethyl) -DL-aspartamide], a new plasma expander . In: Journal of Medicinal Chemistry . tape 16 , no. 8 , August 1, 1973, p. 893-897 , doi : 10.1021 / jm00266a006 .
- ↑ Patent US5142062 : Method for increasing the molecular weight in the manufacture of polysuccinimide. Published on August 25, 1992 , applicant: Röhm GmbH, inventor: J. Knebel, K. Lehmann.
- ↑ a b Patent EU0791616 : Process for producing polysuccinimide and use of said compound. Published on August 27th, 1997 , Applicant: Mitsubishi Chemical Corp., inventor M. Uenaka et al ..
- ↑ Patent US5756595 : Catalytically polymerizing aspartic acid. Published on 26 May 1998 , Applicant: Donlar Corp., Inventor: GY Mazo et al ..
- ↑ Patent US7053170 : Preparation of high molecular weight polysuccinimides. Published May 30, 2006 , Applicant: Aquero Co., Inventor: CS Sikes.
- ↑ Patent EU0612784 : Process for preparing polysuccinimide and polyaspartic acid. Published on August 31, 1994 , Applicant: Bayer AG, inventors T. Groth et al ..
- ↑ Patent US5296578 : Production of polysuccinimide and polyaspartic acid acid from maleic anhydride and ammonia. Published March 22, 1994 , Applicant: Donlar Corp., Inventor: LP Koskan, ARY Meah.
- ↑ Patent US5393868 : Production of polysuccinimide by thermal polymerization of maleamic acid. Published on February 28th, 1995 , Applicant: Rohm and Haas Co., Inventor: MB Freeman et al ..
- ^ KC Low et al .: 6. Commercial Poly (aspartic acid) and Its Uses . In: JE Glass: Hydrophilic Polymers, Advances in Chemistry. 248, 1996, ISBN 978-0-8412-3133-7 , pp. 99-111, doi: 10.1021 / ba-1996-0248.ch006 .
- ↑ G. Swift: Degradable Polymers . 2nd ed. Springer Netherlands, 2002, pp. 379-412, doi : 10.1007 / 978-94-017-1217-0_11 .
- ↑ a b T. Klein: Baypure®, An innovate product family for household and technical applications . 5th Green Chemistry Conference 2003, Barcelona.
- ↑ K. Seo, D. Kim: Design and synthesis of endosomolytic conjugated polyaspartamide for cytosolic drug delivery . In: E. Jabbari, A. Khademhosseini (Eds.): Biologically-responsive hybrid biomaterials: a reference for material scientists and bioengineers. World Scientific Publishing Co., Singapore 2010, ISBN 978-981-4295-67-3 , pp. 191-212, doi : 10.1142 / 9789814295680_0009 .
- ^ Eberhard W. Neuse, Axel G. Perlwitz, Siegfried Schmitt: Water-soluble polyamides as potential drug carriers. III. Relative main-chain stabilities of side chain-functionalized aspartamide polymers on aqueous-phase dialysis . In: Applied Macromolecular Chemistry . tape 192 , no. 1 , November 1, 1991, pp. 35-50 , doi : 10.1002 / apmc.1991.051920103 .
- ↑ Patent US5859179 : Forming superabsorbent polymer. Published January 19, 1999 , Applicant: Solutia Inc., Inventor: Y. Chou.
- ↑ Patent US6072024 : Production process of cross-linked polyaspartic acid. Published on June 6, 2000 , Applicant: Mitsui Chemicals, inventor Y. Irizato et al ..
- ^ Ajay Kumar: Polyaspartic Acid - A Versatile Green Chemical . In: Chemical Sci. Rev. Letters (CSRL) . tape 1 , no. 3 , 2012, ISSN 2278-6783 , p. 162-167 ( PDF ). PDF ( Memento of the original from July 14, 2014 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.



