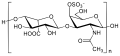
Danaparoid
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mixed polymer of heparan sulfate , dermatan sulfate and chondroitin sulfuric acid | ||||||||||
| General | ||||||||||
| Surname | Danaparoid | |||||||||
| other names |
|
|||||||||
| CAS number |
|
|||||||||
| Monomers / partial structures | Cannot be specified, as a mixture of substances | |||||||||
| ATC code | ||||||||||
| Brief description |
white to almost white, hygroscopic powder |
|||||||||
| Drug information | ||||||||||
| Drug class | ||||||||||
| properties | ||||||||||
| solubility |
easily soluble in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Danaparoid sodium , ( trade name Orgaran ® , manufacturer: Aspen Pharmacare ) is a drug from the group of heparinoids that inhibits blood clotting - similar to heparin . It consists of a mixture of glycosaminoglycans , which are obtained from pig intestinal mucosa . The average molar mass is about 6 kDa.
composition
The medicinal substance consists mainly of heparan sulfate (suleparoid sodium, approx. 84%). Danaparoid sodium also contains dermatan sulfate (sodium salt of chondroitin sulfate B, approx. 12%) and a small amount of chondroitin sulfuric acid ( chondroitin sulfate as free acid, approx. 4%).
Only 4% of the heparan sulfate molecules have a high binding affinity for antithrombin III . Although danaparoid, similar to heparin, is obtained from pig intestinal mucosa, the mixture does not contain any heparin fraction.
application
It is given to prevent deep vein thrombosis in situations where heparin should not be used. These are mostly patients in whom heparin causes a lack of blood platelets , the so-called heparin-induced thrombocytopenia type II (HIT II).
Contraindications
It may not be used, or only after careful consideration, in people who already suffer from a tendency to bleed, uncontrolled high blood pressure , severe kidney or liver failure , stomach or duodenal ulcers or cerebral haemorrhage . If the bleeding is severe, the effect can be reduced by transfusing freshly frozen plasma . A regular check of the number of platelets is necessary.
See also
- Anticoagulation
- Hemostasis
- Thrombus (blood clot)
Web links
- Full label information for ORGARAN ™ (Danaparoid Sodium). (PDF (566 KB)) Food and Drug Administration , June 26, 2001, p. 18 , accessed on October 5, 2010 (English).
- Entry on glycosaminoglycans at Vetpharm, accessed on August 11, 2012.
Individual evidence
- ↑ a b c d data sheet DANAPAROID SODIUM CRS (PDF) at EDQM , accessed on April 27, 2017.
- ^ Entry on danaparoid sodium. In: Römpp Online . Georg Thieme Verlag, accessed on March 18, 2011.
- ↑ Elizabeth M. Van Cott, Massachusetts General Hospital, Boston MA: New Anticoagulants and Heparin-Induced Thrombocytopenia ( Memento from December 26, 2004 in the Internet Archive ) (PDF; 110 kB).
- ↑ Greinacher, A .: Heparin-Induced Thrombocytopenia Dtsch. Doctor bl. 2003; 100: A 2220-2229 (issue 34-35).