Diols


Diols are organic compounds that contain two alcoholic hydroxyl groups (-OH), i.e. dihydric alcohols ( dialcohols ).
An example of a simple, stable diol is ethylene glycol (1,2-ethanediol).
Classification
Alkanediols
Depending on the relative position of the two alcoholic hydroxyl groups in the molecule, a distinction is made between
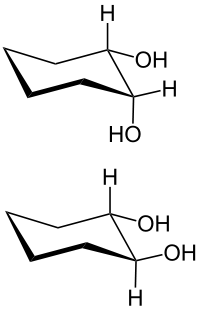
- 1,2-diols ( vicinal ) example: ethylene glycol ( glycols )
- 1,3-diols
- 1,4-diols
Geminal diols, in which the hydroxyl groups are on the same carbon atom, are not stable according to Erlenmeyer's rule and react with elimination of water. However, there are exceptions such as chloral hydrate or ninhydrin .
In 1,2-diols, the z. B. derived from a cycloalkane, a distinction is made between cis -1,2-diols and trans -1,2-diols.
Polyethylene glycols
α, ω-diols formed by the condensation of ethylene glycol, diethylene glycol , triethylene glycol , polyethylene glycol .
Endioles
Endiols (or reductones ) are a subgroup of diols.
Aldehyde hydrates
The formally simplest diol - stable only in aqueous solution - is the aldehyde hydrate of formaldehyde , methanediol . Many textbooks, however, do not count aldehyde hydrates among the diols.
Dihydroxybenzenes
Dihydroxybenzenes are not counted among the diols, just as phenol is not counted among the alcohols.
use
1,2-Ethanediol is used as an antifreeze in water-cooled internal combustion engines and in air conditioning systems. Diols are required in the chemical industry for the production of polyesters , polycarbonates and polyurethanes by means of polycondensation and polyaddition (in the case of polyurethane). Plastic fibers made from polyesters are used in the textile industry under the trade name Diolen .
Individual evidence
- ^ Siegfried Hauptmann : Organic chemistry . 2nd Edition. German publishing house for basic industry, Leipzig 1985, ISBN 3-342-00280-8 , pp. 235-236.
- ^ Brockhaus ABC chemistry. FA Brockhaus Verlag, Leipzig 1965, p. 44.
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 1: A-Cl. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1979, ISBN 3-440-04511-0 , pp. 122-123.