Dibortetrafluoride
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
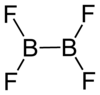
|
||||||||||
| General | ||||||||||
| Surname | Dibortetrafluoride | |||||||||
| other names |
Tetrafluorodiborane |
|||||||||
| Molecular formula | B 2 F 4 | |||||||||
| Brief description |
colorless gas |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 97.61 g mol −1 | |||||||||
| Physical state |
gaseous |
|||||||||
| density |
3.99 g l −1 |
|||||||||
| Melting point |
−56 ° C |
|||||||||
| boiling point |
−34 ° C |
|||||||||
| solubility |
reacts with water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Dibortetrafluoride is an inorganic chemical compound of boron from the group of fluorides .
Extraction and presentation
Dibortetrafluoride can be obtained by the reaction of fluoridation of dibortetrachloride with antimony trifluoride .
In addition to other boron fluorides, it is formed when boron trifluoride is passed over boron at 1900–2000 ° С.
It is also formed during the joint condensation of boron trifluoride and copper atoms . For this purpose, metallic copper is evaporated by electrical heating in a vacuum and the vapor consisting of atomic copper is condensed together with boron trifluoride at −196 ° C.
properties
Dibortetrafluoride is a colorless, flammable gas that reacts with water. Analogously to dibortetrachloride donors, it accumulates to form adducts B 2 F 6 · 2D and slowly decomposes (8% per day at room temperature) into boron trifluoride and a brown solid of the composition BF. In the solid state, dibortetrafluoride has a planar molecule with a valence angle of around 120 °. Single crystals of the compound contain two molecules in a monoclinic unit cell with the space group P 2 1 / n (space group no.14, position 2) and parameters of a = 549 pm, b = 653 pm, c = 483 pm and β = 102, 5 °.
Individual evidence
- ↑ a b c d e f g William M. Haynes: CRC Handbook of Chemistry and Physics, 96th Edition . CRC Press, 2015, ISBN 978-1-4822-6097-7 , pp. 53 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 1033.
- ↑ a b Ralf Steudel : Chemistry of the non-metals with atomic structure, molecular geometry and bond theory . Walter de Gruyter, 1998, ISBN 3-11-012322-3 , p. 554 ( limited preview in Google Book search).
- ↑ Louis Trefonas, William N. Lipscomb: Crystal and Molecular Structure of Diboron Tetrafluoride, B 2 F 4 . In: The Journal of Chemical Physics. 28, 1958, p. 54, doi: 10.1063 / 1.1744079 .

