Dibromopentoxide
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
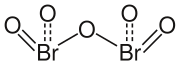
|
|||||||
| General | |||||||
| Surname | Dibromopentoxide | ||||||
| other names |
|
||||||
| Molecular formula | Br 2 O 5 | ||||||
| Brief description |
white needles (as cocrystallisate with three molecules of propionitrile per formula unit) |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 239.81 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
−20 ° C (as cocrystallizate with three molecules of propionitrile per formula unit, decomposition) |
||||||
| solubility |
bad in propionitrile |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Dibromopentoxide or bromine (V) oxide, Br 2 O 5 , is a compound of the elements bromine and oxygen , which is inconsistent under normal conditions, and a representative of halogen oxides . It is a colorless solid that can be isolated at low temperatures and decomposes into its elements from −20 ° C.
presentation
The compound can be obtained by reaction with ozone (ozonation) of elemental bromine at −78 ° C in propionitrile .
properties
Dibromopentoxide is the anhydride of bromic acid and behaves chemically accordingly. Like almost all halogen oxides, bromine (V) oxide has a strong oxidizing effect .
The compound crystallizes from propionitrile in the form of white needles with three molecules of propionitrile per formula unit. From a temperature of −40 ° C dibromopentoxide decomposes into the elements, sometimes explosively. The decomposition point is −20 ° C. In the compound, bromine is in the + V oxidation state and, as expected, has a pyramidal coordination . The double bonds to the terminal oxygen atoms have bond lengths between 160.6 and 161.3 pm . The bonds to the bridging oxygen atom are 187.5 pm and 189.6 pm long. In contrast to the homologous diiodopentoxide (I 2 O 5 ), the terminal oxygen atoms in the dibromopentoxide molecule are aligned and not aligned.
Individual evidence
- ↑ a b c d e f D. Leopold, K. Seppelt: Dibromopentoxide Br 2 O 5 . In: Angew. Chem. 1994, pp. 1043-1044; doi : 10.1002 / anie.19941060921 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.