Iron (III) chloride oxide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ Fe 3+ __ Cl - __ O 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Iron (III) chloride oxide | |||||||||||||||
| other names |
Iron oxychloride |
|||||||||||||||
| Ratio formula | FeOCl | |||||||||||||||
| Brief description |
rust-colored solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 107.30 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
300 ° C (decomposition) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Iron (III) chloride oxide is an inorganic chemical compound of iron from the group of chlorides and oxides .
Extraction and presentation
Iron (III) oxychloride can be obtained by reacting iron (III) chloride hexahydrate with sublimed iron (III) chloride at 250 to 300 ° C. It is free from iron (III) oxide if sublimed iron (III) chloride was used and no higher temperature was used.
properties
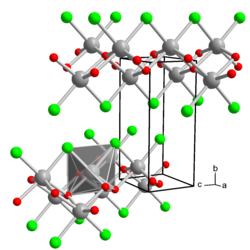
Iron (III) oxychloride is a rust-colored powder made up of small, red needles. Above 300 ° C, disproportionation takes place in iron (III) oxide and iron (III) chloride. It has an orthorhombic crystal structure with the space group Pmmn (space group no. 59) and the lattice parameters a = 375 pm, b = 330 pm and c = 765 pm. The crystal structure consists of layers of double arcs of cis-FeCl 2 O 4 distorted octahedra connected by common edges within the crystallographic ac plane.
Individual evidence
- ↑ a b c d e Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1649.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Duncan W. Bruce, Dermot O'Hare: Inorganic Materials . John Wiley & Sons, 1997, ISBN 0-471-96036-5 , pp. 203 ( limited preview in Google Book search).

