Empagliflozin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
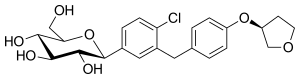
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Empagliflozin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 23 H 27 ClO 7 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 450.91 g mol −1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Empagliflozin (trade name: Jardiance ) is an active ingredient from the group of SGLT-2 inhibitors for the treatment of type 2 diabetes mellitus . It was launched by Boehringer Ingelheim and Eli Lilly and Company .
effect
The mechanism of action of empagliflozin corresponds to that of the structurally related active ingredients dapagliflozin and canagliflozin and is derived from phlorizin ( phloretin ). Empagliflozin selectively inhibits the sodium-glucose cotransporter SGLT2 ( IC 50 = 3.1 nM), which leads to the inhibition of the glucose return transport in the proximal tubules of the kidney and a reduction in the blood glucose level without increasing the insulin secretion. Other sodium-glucose cotransporters are significantly less inhibited.
Benefit assessment
The Federal Joint Committee certified the active ingredient as having an additional benefit.
The Institute for Quality and Efficiency in Health Care had previously seen no additional benefit.
Individual evidence
- ↑ a b Selleck: Empagliflozin (BI 10773) , accessed on December 27, 2019.
- ↑ a b Entry on empagliflozin. In: Römpp Online . Georg Thieme Verlag, accessed on February 8, 2017.
- ↑ Helmut Laschet: Empagliflozin: G-BA sees some considerable additional benefits. In: www.aerztezeitung.de. September 5, 2016, accessed October 13, 2016 .
- ↑ Ulrike Seay, Wolfram Groß, Ulrich Grouven, Thomas Kaiser: A16-46 Empagliflozin - Addendum to commission A16-12. In: www.iqwig.de. September 1, 2016, accessed October 13, 2016 .