Etizolam
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
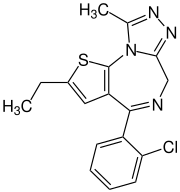
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Etizolam | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 17 H 15 ClN 4 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 342.85 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Etizolam is a benzodiazepine analog from the thienodiazepine group and, like all of its analogs, has amnestic , anxiolytic , anticonvulsant , hypnotic , sedative and muscle-relaxing potential. Due to its special hypnotic potency, it is primarily used for sleep disorders , but can also be used as an anti-anxiety drug .
Etizolam is a very potent thienodiazepine, 1 mg Etizolam is equivalent to about 10 mg diazepam . It has an anxiolytic effect up to six to ten times stronger than diazepam. It quickly floods into the brain and reaches peak concentrations within 30 minutes and two hours. It has a plasma half-life of 3.5 hours, while its active metabolite, alpha-hydroxyetizolam, has a half-life of over 8 hours. The half-life is an important indicator of the intake interval and duration of action.
Etizolam can increase the level of prolactin in the blood. A combination with alcohol and other CNS-effective substances can have incalculable side effects. Like all hypnotics from the benzodiazepine class, Etizolam has a high potential for addiction and abuse. With the 27th Narcotics Amendment Ordinance, Etizolam was made subject to the Narcotics Act in Germany as a prescription narcotic .
distribution
Etizolam is marketed as a research chemical for non-human use. In Italy, Etizolam is used in medicine, namely as Pasaden (produced by Bayer). It is more widespread in Japan and India. In India you can find Etizolam under the trade names Etilaam, Etizola and Etizest. In Japan, Arophalm, Capsafe, Dezolam and Eticalm are well-known trade names.
On the black market one speaks of Etiz, Eitizzy and Etizest. Due to its ready availability as a research chemical, Etizolam is also used as an alternative to alprazolam in the illegal manufacture of "Xanax Bars".
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Patent DE2229845 : Published December 21, 1972 .
- ↑ Niwa T, Shiraga T, Ishii I, Kagayama A, Takagi A: Contribution of human hepatic cytochrome p450 isoforms to the metabolism of psychotropic drugs . In: Biol Pharm Bull . 28, No. 9, September 2005, pp. 1711-1716. doi : 10.1248 / bpb.28.1711 . PMID 16141545 .
- ↑ Mandrioli R, Mercolini L, Raggi MA: Benzodiazepine metabolism: an analytical perspective . In: Curr. Drug metab. . 9, No. 8, October 2008, pp. 827-844. doi : 10.2174 / 138920008786049258 . PMID 18855614 .
- ↑ Lopedota A, Cutrignelli A, Trapani A, et al. : Effects of different cyclodextrins on the morphology, loading and release properties of poly (DL-lactide-co-glycolide) -microparticles containing the hypnotic agent etizolam . In: J Microencapsul . 24, No. 3, May 2007, pp. 214-224. doi : 10.1080 / 02652040601058152 . PMID 17454433 .
- ↑ Depas . Retrieved February 3, 2009.
- ^ Federal Ministry of Health: Cabinet adopts 27th Narcotics Amendment Ordinance . Press release of May 22, 2013, accessed on May 26, 2013.
- ↑ Critical Review Report: etizolam. In: World Health Organization. October 25, 2019, accessed February 19, 2020 .