Eu (hfc) 3
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
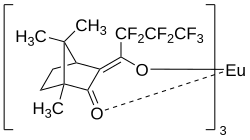
|
||||||||||||||||
| (+) - enantiomer of the compound | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Eu (hfc) 3 | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 42 H 42 EuF 21 O 6 | |||||||||||||||
| Brief description |
dark yellow powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 1193.73 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
156-158 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Eu (hfc) 3 (formerly Eu (hfbc) 3 ) is an enantiomerically pure organic compound of europium , which appears as a dark yellow powder.
use
Eu (hfc) 3 is an optically active lanthanoid shift reagent . It is used to determine the enantiomeric purity of a chiral substance by means of NMR spectroscopy . The compound causes diastereomeric complexes to form, the peaks of which are positioned differently. By integrating related peaks, the diastereomeric ratio can be determined and, as a rule, conclusions can be drawn from this about the enantiomeric ratio .
The compound is used not only as a shift reagent, but also as a catalyst in enantioselective Diels-Alder reactions . A high enantioselectivity is achieved in particular through the use of chiral auxiliaries.
properties
The compound is readily soluble in organic solvents such as dichloromethane or chloroform . The specific angle of rotation is [α] D 20 + 158.0 ° Like many lanthanoid shift reagents (LSR), Eu (hfc) 3 is also a hygroscopic compound.
Eu (hfc) 3 is structurally related to Eu (tfc) 3 and has a perfluorinated alkyl chain two carbon atoms longer on the camphor backbone. In studies, (+) - Eu (hfc) 3 showed a stronger enantiomeric splitting in NMR spectra compared to (+) - Eu (tfc) 3 .
safety instructions
Strong oxidizing agents can cause strong reactions with Eu (hfc) 3 . Hydrogen fluoride , carbon monoxide and carbon dioxide and europium oxides are the decomposition products of the compound.
Individual evidence
- ↑ a b c d Datasheet Europium tris [3- (heptafluoropropylhydroxymethylene) - (+) - camphorate] from Sigma-Aldrich , accessed on February 10, 2019 ( PDF ).
- ↑ Entry on Eu (hfc) 3 . In: Römpp Online . Georg Thieme Verlag, accessed on February 10, 2019.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Lanthanide Shift Reagents. (No longer available online.) In: paperplane.io. Archived from the original on February 12, 2019 ; accessed on February 10, 2019 .
- ↑ Barry M. Trost (Ed.): Additions to C-X bonds . Elsevier , 1992, ISBN 978-0-08-091245-5 , Optical induction in the Eu (hfc) 3 -catalyzed reaction, pp. 682–685 ( limited preview in Google Book Search).
- ↑ Mariane Axt, João Alifantes, Valentim Emílio Uberti Costa: Use of chiral lanthanide shift reagents in the elucidation of NMR signals from enantiomeric mixtures of polycyclic compounds . In: Journal of the Chemical Society Perkin Transactions 2 . tape 0 , no. 12 , 1999, p. 2783-2788 , doi : 10.1039 / A904473F .