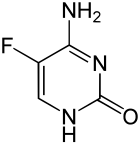
5-fluorocytosine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Flucytosine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 4 H 4 FN 3 O | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
Antifungal agent |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 129.09 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
298-300 ° C (dec.) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
5-fluorocytosine (often also 5-flucytosine ; trade name: Ancotil ) is a heterocyclic organic compound with a pyrimidine backbone. It is a derivative of the nucleobase cytosine with an additional fluorine at position 5. It is used as a systemic antifungal agent for the treatment of mycoses .
pharmacology
5-fluorocytosine is considered a prodrug . It is absorbed into the fungal cell by a cytosine permease and is immediately deaminated to 5-fluorouracil by the cytosine deaminase . The deamination of 5-fluorocytosine to 5-fluorouracil does not take place in the cells of mammals or takes place only to a small extent. It is not known whether 5-fluorocytosine can act as a carcinogen in humans .
5-fluorouracil inhibits DNA synthesis and can lead to the formation of defective RNA, which makes it fungistatic (inhibits the growth of fungi). 5-fluorocytosine works against Candida species (but there are already some resistances here ), Aspergillus species, cryptococci and other fungi. In order to prevent further resistance build-up and because of gaps in the spectrum of activity, it is often combined with amphotericin B (gaps exist for dimorphic fungi, molds, zygomycetes and fusarium). When using 5-fluorouracil, plasma level determinations are required.
Web links
- Entry for Flucytosine in the Human Metabolome Database (HMDB) , accessed March 18, 2019.
Individual evidence
- ↑ a b c data sheet 5-fluorocytosine from Sigma-Aldrich , accessed on December 3, 2013 ( PDF ).
- ^ F. von Bruchhausen: Hager's Handbook of Pharmaceutical Practice: Drugs AK , 5th edition, Springer Verlag, Berlin 1998, ISBN 3-540-61618-7 , p. 226.
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , p. 271.
