Frieze rearrangement
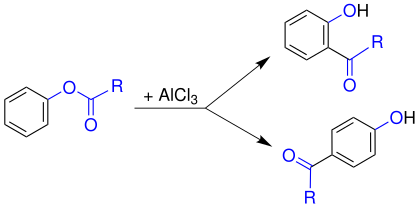
The Fries rearrangement (or Fries shift) is a name reaction in organic chemistry . The reaction is named after its discoverer, the German chemist Karl Fries (1875–1962). The Fries rearrangement here describes the electrophilic rearrangement of aryl esters (e.g., phenyl ester ) by Lewis acid - catalysis to the corresponding aryl ketone.
mechanism
Despite considerable efforts, no conclusive statements on the mechanism have yet been made. Thus, in cross experiments, indications of both intra - and intermolecular processes were found. The course of the reaction depends on both the solvent and the substrate . In general, however, the mechanism below is accepted as the best explanation yet. In a first step, the Lewis acid (here aluminum chloride , AlCl 3 ) attacks the carbonyl oxygen of the acyl group ( 1 ). This is more negative than phenolic oxygen and is therefore a preferred target. This polarizes the bond between the acyl radical and the phenolic oxygen. Then the aluminum chloride migrates to the phenolic oxygen ( 3 ). An acylium cation 5 is released by shifting the binding electrons .
This acylium cation 5 now reacts with the aromatic 4 with classical electrophilic aromatic substitution . An attack in ortho or para position is possible. The orientation of the substitution is temperature dependent. The para product is preferably formed at low temperatures and the ortho product is preferably formed at high temperatures . To restore the aromatic state, a proton is split off after the electrophilic attack and ketone 7 ( ortho substitution) or 10 ( para substitution) is obtained. The resulting ketone remains bound to the aluminum as an anion. The aluminum compound is then hydrolyzed by adding water and the desired hydroxy ketone 8 ( ortho substitution) or 11 ( para substitution) is obtained.
Ortho substitution:
Para substitution:
Photo frieze rearrangement
In addition to the phenyl ester reaction described above, there is a variant known as the Photo-Fries rearrangement, which proceeds via a radical mechanism. It can also be used in the presence of deactivating groups on the aromatic, but due to the generally poor yields, it has so far only been used in the laboratory. If the para position on the phenyl radical is blocked by a substituent (e.g. a methyl group), only ortho hydroxy ketones are formed. If the phenyl radical is not substituted in the para position, mixtures of ortho and para hydroxy ketones are formed. The first step of the mechanism describes the formation of an acyl radical 13 and an aryloxy radical 12 , which are characterized by their mesomeric stabilization.
The mesomeric boundary structures dictate that the acyl radical 13 formed can bind to the aryloxy radical 12 either in the ortho or in the para position . After the rearrangement of a proton , the corresponding hydroxyketone 8 or 11 is formed .
Ortho shift
Para shift
meaning
Since the reaction of phenols with acyl halides under the conditions of Friedel-Crafts acylation yields phenyl esters, but not the desired hydroxyaryl ketones, the reaction is of industrial importance for the synthesis of hydroxyaryl ketones, which are important starting materials for the synthesis of various pharmaceuticals (e.g. Paracetamol or salbutamol ). Instead of aluminum chloride, other Lewis acids ( boron trifluoride , bismuth triflate, etc.) or even strong protic acids ( hydrofluoric acid or methanesulfonic acid ) can also be used in some cases . In order to avoid the consumption of these corrosive and ecologically questionable catalysts, the use of solid-state catalysts is being investigated intensively.
The photochemical Fries rearrangement is an undesirable effect in the light-induced discoloration of inherently colorless plastics. This can be counteracted by installing suitable radical scavengers.
Limits
In all cases, only those esters can be used whose acyl component is stable under the harsh conditions. If the aromatic or acyl component is highly substituted, the yield drops sharply due to excessive steric stress. Deactivating ( meta- directing) groups on the aromatic cause the yields to drop drastically, as is to be expected for a Friedel-Crafts reaction.
literature
- K. Fries, G. Finck: About homologues of coumaranons and their derivatives . In: Reports of the German Chemical Society . tape 41 , no. 3 , October 1, 1908, p. 4271-4284 , doi : 10.1002 / cber.190804103146 .
- K. Fries, G. Finck: About oxygen isologues of homologous indirubins . In: Reports of the German Chemical Society . tape 41 , no. 3 , October 1, 1908, p. 4284-4294 , doi : 10.1002 / cber.190804103147 .
- K. Fries, W. Pfaffendorf: About a condensation product of coumaranone and its conversion into oxine dirubine . In: Reports of the German Chemical Society . tape 43 , no. 1 , January 1, 1910, p. 212-219 , doi : 10.1002 / cber.19100430131 .
- Jerry March: Advanced Organic Chemistry. 3rd Ed., John Wiley & Sons , 1985, ISBN 0-471-88841-9 , pp. 499-500.
Web links
- Frieze rearrangement. www.organic-chemistry.org, accessed March 9, 2009 .
Individual evidence
- ↑ Louis Fieser , Mary Fieser: Organic chemistry . 2nd edition, Verlag Chemie Weinheim, 1972, ISBN 3-527-25075-1 , pp. 926-928.
- ↑ Thomas Laue, Andreas Plagens: Name and Keyword Reactions of Organic Chemistry , 5th Edition, Teubner Study Books Chemistry, 2006, p. 124.
- ↑ a b Zerong Wang: Comprehensive Organic Name Reactions and Reagent (3-Volume Set) Volume 1, Wiley, 2009, ISBN 978-0-471-70450-8 , p. 1143.
- ↑ Jürgen Martens , Klaus Praefcke : Organic sulfur compounds, VII. Photochemical α-cleavage of thiobenzoic acid-Sp-tolylesters in solution . In: Chemical Reports . tape 107 , no. 7 , July 1, 1974, p. 2319-2325 , doi : 10.1002 / cber.19741070716 .
- ↑ Louis Fieser, Mary Fieser: Organic chemistry . 2nd edition, Verlag Chemie Weinheim, 1972, ISBN 3-527-25075-1 , pp. 928-929.