Germanium (II) telluride
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Ge 2+ : __ / Te 2− : __ | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Germanium (II) telluride | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | GeTe | |||||||||||||||
| Brief description |
odorless black solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 200.19 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
6.14 g cm −3 |
|||||||||||||||
| Melting point |
725 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Germanium (II) telluride is a chemical compound from the telluride group .
Extraction and presentation
Germanium (II) telluride can be obtained by reacting germanium with tellurium .
properties
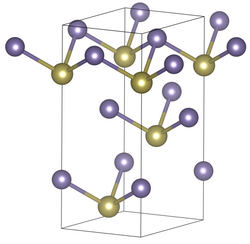
Germanium (II) telluride is an odorless black solid that is insoluble in water. It occurs in three modifications, two forms present at room temperature α (rhombohedral), space group R 3 m (space group no. 160) with the lattice constants a = 4.156 Å , c = 10.663 Å, and γ ( orthorhombic , space group Pnma) and a high temperature form β with a cubic crystal structure of the rock salt type with the space group Fm 3 m (space group no. 225) ). In the case of pure germanium (II) telluride, the α-form is the most common below the ferroelectric Curie temperature of about 670 K, although it depends on the exact stoichiometric composition and tends to the γ-form at 51% tellurium.
use
Germanium (II) telluride is a promising material for thermoelectric applications and an important semiconductor material for prototypical phase change materials for optical and electronic non-volatile memory applications.
Individual evidence
- ↑ a b c d e f g h data sheet germanium (II) telluride at AlfaAesar, accessed on March 27, 2016 ( PDF )(JavaScript required) .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 96th Edition . CRC Press, 2015, ISBN 978-1-4822-6097-7 , pp. 65 ( limited preview in Google Book search).
- ↑ a b E.I. Givargizov, AM Melnikova: Growth of Crystals . Springer Science & Business Media, 2002, ISBN 978-0-306-18121-4 , pp. 12 ( limited preview in Google Book search).
- ↑ Paula Bauer Pereira et al .: Lattice dynamics and structure of GeTe, SnTe and PbTe . In: Physica status solidi B . tape 250 , no. 7 , December 18, 2012, p. 1300-1307 , doi : 10.1002 / pssb.201248412 .
- ↑ R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 476 ( limited preview in Google Book search).
- ↑ M. Bardosova, T. Wagner: Nanomaterials and Nanoarchitectures A Complex Review of Current Hot Topics and their Applications . Springer, 2015, ISBN 978-94-017-9921-8 , pp. 157 ( limited preview in Google Book search).
- ↑ Data sheet Germanium (II) telluride, 99.999% trace metals basis from Sigma-Aldrich , accessed on March 27, 2016 ( PDF ).
