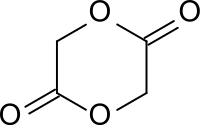
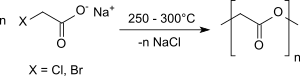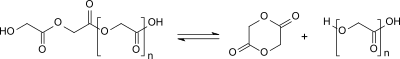Glycolide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Glycolide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 4 O 4 | |||||||||||||||
| Brief description |
colorless flakes or solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 116.07 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.6125 g cm −3 at 22.5 ° C |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Glycolide is the dimeric cyclic ester of glycolic acid (hydroxyacetic acid), which can not form lactone due to the close proximity of the hydroxyl and carboxy groups. The dimeric linear ester of glycolic acid is a 5- hydroxycarboxylic acid , which polymerizes further to form low molecular weight glycolic acid oligomers.
The six-membered ring structure of the glycolide is formed from this through thermal depolymerization . The ring-opening polymerization produces polyglycolide (polyglycolic acid PGA) with molar masses M w > 50,000 from glycolide , which is used as a biodegradable implant material and in industrial applications e.g. B. is used as a barrier film with reduced oxygen and carbon dioxide permeability for polyethylene terephthalate beverage and food packaging.
Extraction and presentation
The production of glycolide by heating glycolic acid in vacuo was first described in 1860. Formally, two molecules of glycolic acid react with elimination of water to form the monoester, which cyclizes with further elimination of water to form glycolide. In fact, oligomeric polyglycolic acid (M w <30,000) is formed on heating , from which glycolide is formed by depolymerization from the hydroxyl group end.
When alkali salts of haloacetic acids are heated dry under nitrogen in a vacuum to temperatures of 250 ° C to 300 ° C, glycolide is formed in modest yields of approx. 20%.
In the presence of copper chips for better temperature transfer in the solid mixture, however, yields of up to 72% are achieved.
Thermal depolymerization of the oligomeric polyglycolic acid obtained by polycondensation of glycolic acid in the presence of tin compounds as transesterification catalysts has established itself as a process for the production of glycolide which can also be used on an industrial scale . An equilibrium is established between ring-shaped glycolide and chain-shaped oligoglycolic acid, from which glycolide is continuously removed by vacuum distillation.
For this purpose, the commercially available 70% aqueous glycolic acid solution is first heated to 220 ° C. under normal pressure and then under vacuum. The oligoglycolic acid obtained is in high-boiling polar solvents, such as. B. dissolved oligoethylene glycol diethern and heated with tin catalysts under vacuum to 230 ° C, so that glycolide is distilled off together with the solvent and crystallized in high purity and yield on cooling.
properties
Pure 1,4-dioxane-2,5-dione is a white crystalline solid that crystallizes in flakes and melts at 86 to 87 ° C. Through a combination of multiple recrystallization and subsequent sublimation, glycolide is obtained in purities (> 99.9%) that consistently yield high molecular weight polymers in ring-opening polymerization.
Applications
1,4-Dioxane-2,5-dione can be used in a ring-opening polymerization as a dimeric cyclic ester both cationically and anionically, preferably under catalysis with organic tin compounds, such as. B. tin dioctoate (tin (II) -2-ethylhexanoate), or with basic alkoxides, such as. B. aluminum isopropoxide in an intramolecular transesterification to polyglycolide (polyhydroxyacetic acid or polyglycolic acid PGA) are polymerized.
PGA is a crystalline, thermoplastic and biodegradable polymer with high mechanical stability and interesting barrier properties for industrial and medical applications.
Individual evidence
- ↑ a b c d e f C.A. Bischoff, P. Walden: About derivatives of glycolic acid . In: Liebigs Ann. Chem. Band 279 , no. 1-2 , 1894, pp. 45-70 , doi : 10.1002 / jlac.18942790106 .
- ↑ a b R.C. Weast: CRC Handbook of Chemistry and Physics, 61st Edition . CRC Press, Inc., 1980, ISBN 0-8493-0461-X , pp. C-343 .
- ↑ a b c d data sheet Glycolide at Sigma-Aldrich , accessed on December 23, 2019 ( PDF ).
- ^ CL Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier, 2014, ISBN 978-0-323-28659-6 , pp. 269 .
- ↑ C.-C. Lin, KS Anseth: Chapter II.4.3. The biodegradability of biodegradable polymeric biomaterials in Handbook of Biodegradable Polymers . Ed .: BD Ratner, AS Hoffman, FJ Schoen, JE Lemons. Academic Press, 2012, ISBN 978-0-12-374626-9 , pp. 716-727 .
- ↑ KUREDUX (R) , Polyglycolic Acid (PGA) Resin. (PDF).
- ^ W. Heintz: Pogg. Ann. Chem. U. Phys. tape 109 , 1860, p. 484 .
- ^ F. Andreas, R. Sowada, J. Scholz: Representation and properties of glycolide . In: J. Prakt. Chem. Band 18 , no. 3-4 , 1962, pp. 141-149 , doi : 10.1002 / prac.19620180305 .
- ↑ CA Bischoff, P. Walden: Ueber das Glycolid and its homologues . In: Ber. German chem. Ges. Band 26 , no. 1 , 1893, p. 262-265 , doi : 10.1002 / cber.18930260158 .
- ↑ A. Sporzynski, W. KOÇAY, HVA Briscoe: A new method of preparing glycollide . In: Rec. Trav. Chim. Pays-Bas . tape 68 , no. 7 , 1949, pp. 613-618 , doi : 10.1002 / recl.19490680705 .
- ↑ Patent US8722908B2 : Method for producing glycolide. Filed December 16, 2010 , published May 13, 2014 , Applicant: Kureha Corp., Inventors: S. Suzuki, K. Yamane, M. Kagoshima, M. Kikuchi.
- ↑ Patent US3597450 : Preparation of glycolide polymerizable into polyglycolic acid of consistently high molecular weight. Filed November 5, 1969 , published August 3, 1971 , Applicant: American Cyanamid Co., Inventors: EE Schmitt, RA Polistina, M. Epstein, DA DeProspero.
- ↑ H. Amine, O. Karima, BM El Amine, M. Belbachir, R. Meghabar: Cationic Ring Opening Polymerization of Glycolide Catalysed by a Montmorillonite Clay Catalyst . In: J. Polym. Res. Band 12 , no. 5 , 2005, p. 361-365 , doi : 10.1007 / s10965-004-0004-1 .
- ↑ a b O. Dechy-Cabaret, B. Martin-Vaca, D. Bourissou: Controlled ring-opening polymerization of lactide and glycolide . In: Chem. Rev. Band 104 , no. 12 , 2004, p. 6147-6176 , doi : 10.1021 / cr040002s .
- ↑ S. Dutta, W.-C. Hung, B.-H. Huang, C.-C. Lin: Recent developments in metal-catalyzed ring-opening polymerization of lactides and glycolides: Preparation of polylactide, polyglycolide, and poly (lactide-co-glycolide) . In: Adv. Polym. Sci. tape 245 . Springer, 2012, ISBN 978-3-642-27153-3 , pp. 219-284 , doi : 10.1007 / 978-3-642-27154-0 .
- ↑ KUREDUX (R) , Polyglycolic Acid (PGA) Resin, For Packaging and Industrial Applications (PDF).