Gold (I) iodide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
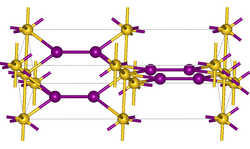
|
||||||||||||||||
| __ Au + __ I - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Gold (I) iodide | |||||||||||||||
| other names |
Gold monoiodide |
|||||||||||||||
| Ratio formula | AuI | |||||||||||||||
| Brief description |
yellow solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 323.87 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
8.25 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
120 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Gold (I) iodide is an inorganic chemical compound of gold from the group of iodides .
Extraction and presentation
Gold (I) iodide can be obtained by reacting a tetrachloridoauric acid solution with potassium iodide . It is also possible to manufacture by reacting gold and iodine in a protective atmosphere at around 390 ° C.
properties
Gold (I) iodide is a yellow, crystalline powder that gradually decomposes on contact with water, humidity or light. It has a tetragonal crystal structure with the space group P 4 2 / ncm (space group no. 138) , a = 4.359 Å , c = 13.711 Å.
Individual evidence
- ↑ a b c d e f data sheet Gold (I) iodide, 99.9% trace metals basis from Sigma-Aldrich , accessed on April 4, 2013 ( PDF ).
- ↑ a b c d Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 1014.
- ^ Dale L. Perry: Handbook of Inorganic Compounds, Second Edition . Taylor & Francis US, 2011, ISBN 1-4398-1462-7 , pp. 486 ( limited preview in Google Book search).
