Herapathite
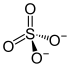
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
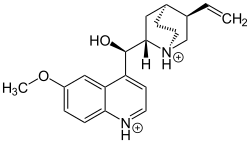 |
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Herapathite | ||||||||||||||||||
| other names |
Quinine iodosulfate hexahydrate |
||||||||||||||||||
| Molecular formula | C 80 H 104 I 6 N 8 O 20 S 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 3084.57 g mol −1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Herapathite is a chemical compound that has polarizing properties.
history
Herapathit was discovered in an unusual way in 1852 by the English doctor and researcher William Bird Herapath (1820–1868). In a biological test, his assistant dripped tincture of iodine into dog urine after it had been given quinine . Green, shimmering, needle-shaped crystals formed in the dog's urine , which aroused the researcher's interest. During examinations under the microscope, he found strong polarization properties of the substance, which he concluded from the black coloration when the crystals overlapped at certain angles. The chemical structure was clarified by Sophus Mads Jørgensen in 1876 . The compound named after its discoverer is used for the production of polarization filters . Initial problems with too small a size of the crystals could be countered by improved crystal growing methods (according to Ferdinand Bernauer , who succeeded in growing large-area, but only fractions of a millimeter thick single crystals) or by methods for the same dichroic alignment of a large number of small crystals. In the 1930s, for example, the American physicist Edwin Herbert Land developed polarization films that consisted of stretched plastic films (with which the molecules of the plastic also aligned themselves in parallel) with embedded herapathite crystals. These polarizing filters are still used today in photography, but also in sunglasses . The patent for this technology was granted to the physicist in 1933, who a little later founded the company Polaroid named after the film . During the same period, Charles West established the orthorhombic crystal structure . But it was not until 2009 that chemists from the University of Washington in Seattle deciphered the exact structure of the herapathite using X-ray diffraction analyzes.
properties
Chemically, Herapathit is quinine sulfate triiodide (or iodoquinine sulfate or quinine bisulfate polyiodide or quinine hydrogen sulfate polyiodide) with the chemical formula 4 (C 20 H 24 N 2 O 2 ) H 2 3 (SO 4 ) 2 (I 3 ) · 6 · (H 2 O), whereby several different crystals can be produced with these components through the different oxidation states of iodine, but all of them have similar properties to herapathite. Herapathit is produced by dissolving quinine sulfuric acid in acetic acid with the addition of iodine. The falling needle-shaped crystals are colorless, but in the incident light they have a magnificent green metallic sheen. Herapathit is sparingly soluble in water and easily soluble in alcohols . It polarizes light five times more than tourmaline and is used in polarizing filters. The color properties of the dichroic material are created by iodide anion chains.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Wolfgang Baier: Source representations for the history of photography . 2nd edition, Schirmer / Mosel, Munich 1980, ISBN 3-921375-60-6 , p. 323.
- ↑ EW Thulstrup, J. Michl: Elementary Polarization Spectroscopy. 1st edition, John Wiley & Sons, 1997, ISBN 0-471-19057-8 , p. 1.
- ↑ a b S. M Jörgensen: About the so-called Herapathit and similar Acidperiodide . In: Journal for Practical Chemistry . tape 14 , no. 1 , 1876, p. 213-268 , doi : 10.1002 / prac.18760140113 .
- ^ Martin Grabau: Polarized Light Enters the World of Everyday Life . In: Journal of Applied Physics, Volume 9, April 1938, No. 4, p. 217
- ↑ Bart Kahr, John Freudenthal, Shane Phillips, Werner Kaminsky: Herapathite . In: Science . tape 324 , no. 5933 , 2009, pp. 1407 , doi : 10.1126 / science.1173605 .
- ↑ Uta Bilow: A well-known mineral in a new light . FAZ.NET, June 7, 2009.
- ↑ Herapathit . In: Meyers Konversations-Lexikon. 1888.
- ^ CD West: Crystallography of herapathite. In: American Mineralogist. Vol. 22, No. 5, 1937, p. 7310 (PDF).
- ↑ On the representation of large crystals of sulfuric acid iodine-quinine (herapathite), which can be used as tourmalines for optical purposes . In: Annals of Physics . tape 166 , no. 12 , 1853, pp. 616–622 , doi : 10.1002 / andp.18531661211 ( PDF ).