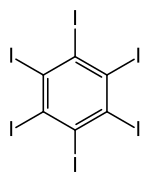
Hexaiodbenzene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hexaiodbenzene | |||||||||||||||
| other names |
Periodbenzene |
|||||||||||||||
| Molecular formula | C 6 I 6 | |||||||||||||||
| Brief description |
orange needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 833.49 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
430 ° C (dec.) |
|||||||||||||||
| solubility |
moderately in N -methyl-2-pyrrolidone |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Hexaiodbenzene (C 6 I 6 ) is an aromatic compound. The molecule is as benzene constructed, except that all hydrogen -atoms by iodine -atoms replaced were.
Extraction and presentation
The compound was first prepared by iodination of benzoic acid in the presence of hot fuming sulfuric acid . Otherwise, it is prepared from benzene with periodic acid and potassium iodide by stirring in sulfuric acid at 0 ° C and then heating to 100 ° C with a yield of around 73%. The result of an earlier synthesis attempt by Levitt and Iglesias with the same reagent identified Mattern as 1,2,4,5-tetraiodobenzene with a melting point of 249-252 ° C.
properties
Physical Properties
Hexaiodbenzene forms orange needles and is practically insoluble in water. It is rather sparingly soluble in N -methyl-2-pyrrolidone and dimethyl sulfoxide . It melts at 430.degree. C., a certain decomposition with splitting off of I 2 being observed from 370.degree.
Crystallographic properties
The structure of hexaiodbenzene was clarified by X-ray structure analysis . The crystals are monoclinic, pseudohexagonal, space group P2 1 / c, according to which the C 6 I 6 unit is centrally symmetric. The carbon framework is a regular, flat hexagon with a mean C – C distance of 141 ± 5 pm . The iodine atoms are in close proximity to this plane, they show small shifts (approx. 4 pm) alternately above and below the middle plane of the ring. The mean intra- molecular I – I distance is 350.7 ± 0.3 pm. The shortest intermolecular distance is similar (376 pm), and surprisingly short in comparison with twice the van der Waals radius (430 pm). The C – I distance is 209.3 ± 3.7 pm.
The crystal and molecular structures are retained even under high pressure of up to 9.7 GPa.
Individual evidence
- ↑ a b c d e f g h Daniell Lewis Mattern: Periodination of Benzene with Periodate / Iodide , in: J. Org. Chem. , 1983 , 48 (24), pp. 4772-4773 ( doi : 10.1021 / jo00172a063 ; PDF ).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Erwin Rupp: About the perhalogenated phthalic acids and the hexajodbenzene , in: Chem. Ber. , 1896 , 29 , pp. 1625-1634 ( doi : 10.1002 / cber.18960290293 ).
- ↑ LS Levitt, R. Iglesias: The Periodination Reaction: Fast One-Step Synthesis of C 6 I 6 from C 6 H 6 , in: J. Org. Chem. , 1982 , 47 (24), pp. 4770-4770 ( doi : 10.1021 / jo00145a032 ).
- ↑ Rosemary J. Steer, SF Watkins, P. Woodward: Crystal and Molecular Structure of Hexaiodobenzene , in: J. Chem. Soc. C , 1970 , pp. 403-408 ( doi : 10.1039 / J39700000403 ).
- ↑ Nakayama, Atsuko; Fujihisa, Hiroshi; Aoki, Katsutoshi; Carlón, Raquel Pérez: Structural Study of Hexaiodobenzene up to 9.7 GPa , in: Physical Review B (Condensed Matter and Materials Physics), 2000 , 62 (13), pp. 8759-8765 ( doi : 10.1103 / PhysRevB.62.8759 ).
literature
- TA Babushkina, TL Khotsyanova GK Semin: Crystal Structure of Hexabromo- and Hexaiodobenzene and the NQR Spectra of Br 79 and I 127 in these Compounds , in: Journal of Structural Chemistry , 1965 , 6 (2), pp. 285–286 ( doi : 10.1007 / BF00745958 ) (translated from: Zhurnal Strukturnoi Khimii, Vol. 6, No. 2, pp. 307-308, March-April, 1965).
- Masako Suzuki: Vibrational Spectra of Hexabromobenzene and Hexaiodobenzene , in: Spectrochimica Acta Part A: Molecular Spectroscopy, 1977 , 33 (10), pp. 921-927 ( doi : 10.1016 / 0584-8539 (77) 80092-5 ).
- DJ Sagl, JC Martin: The Stable Singlet Ground State Dication of Hexaiodobenzene: Possibly a σ-Delocalized Dication , in: J. Am. Chem. Soc. , 1988 , 110 (17), pp. 5827-5833 ( doi : 10.1021 / ja00225a038 ).
- S. Ghosh, CM Reddy, GR Desiraju: Hexaiodobenzene: a redetermination at 100 K , in: Acta Crystallographica , Section E , Structure Reports Online, 2007 , 63 (2), o910 – o911 ( doi : 10.1107 / S1600536807002279 ).