Hop bitter substances
Hop bitter substances determine the characteristic bitterness of beer . The entire group of hop bitter substances has sedating , antibiotic and estrogenic properties. It is divided in the from humulone efferent "humulones" and in the "lupulones", the descendants of the Lupulons ( β-Lupulinsäure group). Hop bitter substances are very unstable; Various compounds are formed through oxidative degradation, including 2-methylbut-3-en-2-ol , for which sedating effects were found in animal experiments. Hop cones (hop flowers) and hop glands (lupulin) are pharmaceutical drugs used as mild sedatives .
General
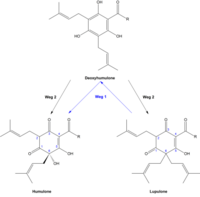
The bitter substances come from the glandular hairs (glandular glands, lupulin glands at the lower end of the bracts) of the female flowers ( hop cones) of hops ( Humulus lupulus ), which contain, among other things, a resinous excretion. A distinction is made between hard resin and soft resin , whereby soft resin is readily soluble in hexane, whereas hard resin is not. The soft resin can be further in α - and β divide -Weichharz. The α -soft resin consists primarily of the humulones ( α- acids). The amount of α- acids depends on the variety, provenance, vintage, the time of harvest, the method of treatment and the age of the hops. The β -soft resin contains the lupulones ( β- acids).
The resin in the air-dried hop cones is made up as follows:
Humulones
The humulones of α -Weichharzes consist mainly of humulone ( α -Lupulinsäure) and deviated from its derivatives, the so-called. Co-, pre-, adsorption, and Adpre- Posthumulonen.
properties
Chemical properties
Structurally, humulones are derivatives of phloroglucinol (1,3,5-trihydroxybenzene), with two isoprenoid side chains generally occurring at C2 and C6 , an additional OH group at C6 and an acyl radical at C4. The two residues at C6 abolish the aromatic basic structure of phloroglucin and two of the originally three OH groups of the basic body are present as keto functions. In addition, a stereogenic center arises at C6. The humulones differ in their different residues on the side chain at C4.
| α-acids | Remainder R | [%] |
|---|---|---|
| Humulone | CH 2 CH (CH 3 ) 2 | 35-70 |
| Cohumulone | CH (CH 3 ) 2 | 20-65 |
| Adhumulon | CH (CH 3 ) CH 2 CH 3 | 10-15 |
| Prehumulon | CH 2 CH 2 CH (CH 3 ) 2 | 1-10 |
| Posthumulon | CH 2 CH 3 | 1-3 |
The humulone and its derivatives have a pK s value of 4.7 to 5.7 and, therefore, in water weakly dissociated. They are therefore weak acids that have a low solubility in water. At a pH value of 5.9, 480 mg / L of the humulones dissolve, at pH 5.0 only 40 mg / L at 25 ° C and 60 mg / L at 100 ° C. Since during the brewing process, z. B. during fermentation, a pH drop is caused, the humulones would not pass into the drink, or only in very small quantities. In order to increase the solubility of the humulones, they are converted into their isomers, the isohumulones , when the wort is boiled .
The humulones are very labile substances that can easily be converted into a large number of secondary products through heat, atmospheric oxygen or storage. For example, through ring narrowing, the six-membered humulone is formed into isohumulone, which is made up of a five-membered ring. Isohumulones also have a higher bitter value. These changes happen easily because they are volatile, sensitive bodies due to their constitution. The presence of the β-tetracarbonyl system and its tautomers (in the case of humulones by enolization at C5) causes the sensitivity in both alkaline and acidic solutions.
Bacteriostatic effect
The humulones have a bacteriostatic activity against gram-positive bacteria, this being based on interactions between the isoprenyl groups of the bitter substances and the cell plasma membrane of the bacteria. The more isoprenyl groups a bitter substance molecule has, the more bacteriostatic the effect. They act z. B. against the causative agent of tuberculosis .
Anti-inflammatory effect
An anti-inflammatory effect of humulone was proven, in which there is a suppression of the transcription of the gene belonging to cyclooxygenase-2 (COX-2), whereby the formation of prostaglandins is inhibited.
Analytics
The reliable determination of the individual active ingredients is achieved by spectroscopic methods and by coupling HPLC with mass spectrometry . These methods are supplemented by sensory processes.
Reactions
Biochemical reactions
The biosynthetic pathway of humulones and lupulones has not yet been fully clarified. One possible theory is that during biogenesis, first the lupulones are formed and from these the humulones are created via the deoxyhumulone (path 1). According to another theory, the biosynthetic pathway should run via deoxyhumulone as a common precursor of humulones and lupulones (path 2). Since the quotient of lupulones to humulones decreases during the ripening phase, it can be assumed that first the lupulones and only then the humulones are formed and thus path 1 is the more important.
Follow-on products
Through drying, storage, processing and wort boiling during the brewing process, the humulones and lupulones are converted into a large number of secondary products. These differ greatly in the intensity of the bitter taste.
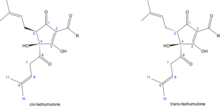
The five-ring system of isohumulones is formed by the ring narrowing of the six-membered ring of humulones.
The mechanism consists of an oxa-di-π-methane rearrangement followed by an opening of the resulting cyclopropanol ring.
The corresponding cis and trans isohumulones can be created from the respective humulones . In the cis isohumulones, the OH group on C4 and the isoprenyl residue on C5 point to the same side of the molecule. In the case of the trans isohumulones, these are in the opposite direction.
There are noticeable differences between the stereoisomers . The cis -isohumulones (half-life> 5 years) have a significantly higher stability than the corresponding trans -isomers (half-life approx. 1 year). The ratio in which the cis / trans isomers are formed depends on the reaction conditions. However, twice as many cis as trans isohumulones are normally formed in the wort medium.
Isohumulones are significantly more bitter and more soluble than their starting materials. During the brewing process it is therefore desirable to convert the humulones into their isomers. The taste threshold of isohumulones is approximately 6 mg / L. They form the quantitatively most significant part with regard to the bitter taste of beers. Beers often contain between 15 mg / L (American lager beers) and 100 mg / L (English ales) of these compounds. The content in wheat beers is often below 10 mg / L.
Beer is usually only available in colored bottles in order to avoid the light taste due to the decomposition of the hop bitter substances into 3-methyl-2-buten-1-thiol , which is based on a photolytic reaction , with riboflavin serving as a catalyst. Brown bottles have a measurably better filter effect than green or white bottles.
The 3-methylbutenyl radical can be split off from isohumulone by α cleavage at the (R-CO) bond - Norrish type I reaction . This releases thiols such as 3-methyl-2-buten-1-thiol (MBT) from naturally occurring sulfur compounds ( proteins ) in beer , which give the beer an unpleasant taste and animal smell (lightstruck / skunky flavor). The release of the undesired MBT can be prevented by converting the isohumulones into their reduced forms. The carbonyl group at C6 or the C = C double bond at C8 of the isohexenyl side chain is reduced, which presumably prevents the formation of the 3-methylbutenyl radical. The reduced isohumulones are more stable and are characterized by a z. T. greater bitterness and improved foam stability (see Table 1). They can also be added later to the brewing process and, according to a study, are not considered to be harmful to health.
| Relative bitterness | Ability to stabilize foam | |
|---|---|---|
| Isohumulones | 1.0 | xx |
| Dihydro-Isohumulone (Rho-) | 0.6-0.7 | xx |
| Tetrahydro-isohumulones | 1.5-1.9 | xxx |
| Hexahydro-isohumulones | 1.0-1.2 | xxxx |
Application in industry
In the traditional brewing process, dried hop cones are added to the wort and cooked in the wort kettle for at least an hour . The humulones are extracted and converted into their isomers, the isohumulones. However, the yield of the bitter substances from the hops in the traditional brewing process is poor, and typically only about 30% of the humulones present in the hops pass into the beer as isohumulones. Nowadays, hop pellets are mostly used instead of dried hop cones. These consist of ground hops, which are pressed into pellets and packed in an inert gas atmosphere. The hop pellets have the advantage of better shelf life, fewer oxidation reactions and a smaller volume. The efficiency with which beer can be bittered with hops can be increased by a separate extraction of the humulones from the hops with supercritical carbon dioxide or ethanol . Therefore, in addition to hops, you will also find hop extract on the list of ingredients on many beer bottles.
Process engineering application in industry
Nowadays the hops are first extracted with supercritical CO 2 . The extract contains humulones, lupulones, hop oils and some low molecular weight fats and waxes . The humulones contained in the extract can be converted into isohumulones in an aqueous suspension using heat, alkali and magnesium ions, which accelerate the isomerization reactions. The isohumulones are separated from the reaction mixture and purified by fractional precipitation steps using mineral acids. Using isohumulones prepared in this way, the yield of the original humulones can be improved up to 80%. In most cases, a significant reduction in the cost of bittering beer is also noted. However, this procedure does not comply with the German Purity Law .
Lupulones
| β-acids | Remainder R | [%] |
|---|---|---|
| Lupulon | CH 2 CH (CH 3 ) 2 | 35-55 |
| Colupulon | CH (CH 3 ) 2 | 20-55 |
| Adlupulon | CH (CH 3 ) CH 2 CH 3 | 10-15 |
| Prelupulon | CH 2 CH 2 CH (CH 3 ) 2 | 1-3 |
| Post lupulon | CH 2 CH 3 | ? |
The β -soft resin contains lupulone ( β- lupulinic acid) as the main component and its derivatives, the co-, pre-, ad- and post-lupulones, as secondary components. Structurally speaking, lupulones are also derivatives of phloroglucinol with three isoprenoid side chains that carry an acyl group .
They differ from humulones in that the hydroxyl group at the stereogenic center C6 of the humulone is replaced by an additional isoprene unit. The lupulones are therefore less polar than the humulones and have an even lower water solubility. At a pH value between pH 5–6, only 1.5 mg / L of lupulones dissolve at 25 ° C and 9 mg / L at 100 ° C. Like humulones, lupulones are converted into bitter-tasting compounds. Some of the products derived from Lupulone, Hulupone or Luputrione have a particularly pleasant, mild bitter taste. However, they are much less bitter and much less soluble, so that the bitter taste of the beer is mainly determined by the humulone fraction. However, many other oxidation products of lupulones have an unpleasant taste instead, so that low-lupulone hops are often used for brewing beer. Accordingly, they are less important for the beer industry.
Individual evidence
- ↑ R. Hegnauer: Chemotaxonomy of plants. Volume 3: Dicotyledoneae: Acanthaceae - Cyrillaceae. Birkhäuser, 2014, ISBN 978-3-7643-0166-8 , p. 353.
- ↑ a b c d e f g h i j k Max Wichtl : Hops (Humulus Lupulus): Still an important component of herbal sedatives. In: Switzerland. Zschr. Holistic Medicine. 17, 2005, pp. 95-99. Publishing house for holistic medicine, Basel. www.ganzheits-medizin.ch.
- ↑ Johann Maier: To the knowledge of the hop bitter substances 4-deoxy-humulon and α + β -soft resin. Dissertation, TH Munich. Freising-Weihenstephan 1962, p. 6 ff.
- ↑ Jana Nagel: EST analysis of Humulus lupulus L. trichomes - identification of an O -methyltransferase, which catalyzes the biosynthesis of xanthohumol. Dissertation . Halle (Saale) 2009, p. 6.
- ↑ a b c d e f g h i j k l Ludwig Narziss , Werner Back: Die Bierbrauerei. Volume 2: The technology of wort preparation. 8., revised. and exp. Edition. Wiley-VCH, Weinheim 2009, ISBN 978-3-527-32533-7 , p. 63 ff.
- ↑ a b c d e f g H. D. Belitz, W. Grosch, P. Schieberle: Textbook of food chemistry. 6th, completely revised edition. Springer-Verlag, Berlin / Heidelberg 2008, ISBN 978-3-540-73201-3 , p. 923 ff.
- ↑ Johannes Beier: About the biosynthesis of hop bitter substances. Dissertation. TU Munich, 1973, p. 3 f.
- ↑ a b c d e f g h i j Denis de Keukeleire: Fundamentals Of Beer And Hop Chemistry. In: Química Nova. Vol. 23, No. 1, São Paulo Jan./Feb. 2000.
- ↑ a b c d e f g h i j Richard JH Wilson, Robert Smith: Patent DE69934830T2; Process for the hydrogenation of hop resin acids. S. 5. Accessed June 2, 2014.
- ↑ a b c d e f Ludwig Narcissus, Werner Back: The beer brewery. Volume 2: The technology of wort preparation. 8., revised. and exp. Edition. Wiley-VCH, Weinheim 2009, ISBN 978-3-527-32533-7 , p. 269.
- ↑ a b Johann Maier: To the knowledge of the hop bitter substances 4-deoxy-humulon and α + β-soft resin. Dissertation, TH Munich. Freising-Weihenstephan 1962, p. 2.
- ↑ K. Yamamoto, J. Wang, S. Yamamoto, H. Tobe: Suppression of Cyclooxygenase-2 Gene Transcription by Humulone. In: Kenneth V. Honn, Lawrence J. Marnett, Santosh Nigam, Edward Dennis, Charles Serhan (Eds.): Eicosanoids and other bioactive lipids in cancer, inflammation, and radiation injury. Volume 5, Springer, 2002, ISBN 0-306-47283-X , pp. 73-76.
- ↑ M. Dresel, A. Dunkel, T. Hofmann: Sensomics analysis of key bitter compounds in the hard resin of hops (Humulus lupulus L.) and their contribution to the bitter profile of Pilsner-type beer. In: J Agric Food Chem. 63 (13), Apr 8, 2015, pp. 3402-3418. PMID 25793563
- ↑ M. Dušek, J. Olšovská, K. Krofta, M. Jurková, A. Mikyška: Qualitative determination of β-acids and their transformation products in beer and hop using HR / AM-LC-MS / MS. In: J Agric Food Chem. 62 (31), Aug 6, 2014, pp. 7690-7697. PMID 25099125
- ↑ a b c d e Birgit Witte: Photochemical and thermal annulation reactions of conjugated alenylcyclohex-2-enones . Dissertation. Hamburg 1999, p. 5 f.
- ↑ a b Stefan Hanke: Investigations into the influence of hop technology on the taste stability and harmony of bottom-fermented beers . Dissertation. Technical University of Munich, 2009, p. 42.
- ^ CI Chappel, SY Smith, M. Chagnon: Subchronic Toxicity of Tetrahydroisohumulone and Hexyhydroisohumulone in the Beagle Dog. In: Food and Chemical Toxicology . 36, Elsevier 1998, p. 915 ff.
- ↑ Gesa Haseleu, Daniel Intelmann, Thomas Hofmann: Structure determination and sensory evaluation of novel bitter compounds formed from β-acids of hop (Humulus lupulus L.) upon wort boiling. In: Food Chemistry . 116, Elsevier 2009, pp. 71-81.