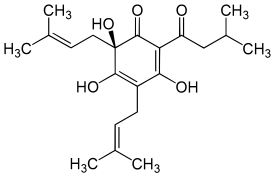
Humulone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Humulone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 21 H 30 O 5 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 362.47 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
66 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Humulone (also α-lupulinic acid ) is a bacteriostatic bitter substance .
Occurrence
The resin of the ripe hops Humulus lupulus contains humulone. Its rearrangement products (especially cis - and trans -isohumulone), which are created by heating, give the beer its characteristic bitter taste. It is counted among the hop bitter substances .
Structurally, humulone is a hydroxyphloroglucine with three isoprenoid side chains. An anti-inflammatory effect of humulone was shown to suppress the transcription of the gene belonging to cyclooxygenase-2 ( COX-2 ), which inhibits the formation of prostaglandins .
Web links
Individual evidence
- ↑ Entry on Humulones. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Briggs, DE, CA Boulton, PA Brookes, and R. Stevens, Brewing Science and Practice. 2004, Cambridge, UK: Woodhead Publishing Limited, ISBN 978-0-8493-2547-2 .
- ↑ K. Yamamoto, J. Wang, S. Yamamoto, H. Tobe: Suppression of Cyclooxygenase-2 Gene Transcription by Humulone. In: Kenneth V. Honn, Lawrence J. Marnett, Santosh Nigam, Edward Dennis, Charles Serhan (Eds.): Eicosanoids and other bioactive lipids in cancer, inflammation, and radiation injury, Volume 5. Springer, 2002, ISBN 978-0 -306-47283-1 , pp. 73-76.

