Bis (hydroxylammonium) sulfate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Bis (hydroxylammonium) sulfate | |||||||||||||||
| other names |
Hydroxylammonium sulfate |
|||||||||||||||
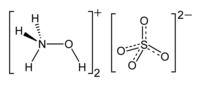
| Molecular formula | (NH 3 OH) 2 SO 4 | |||||||||||||||
| Brief description |
colorless to white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 164.14 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.88 g cm −3 |
|||||||||||||||
| Melting point |
> 120 ° C (decomposition) |
|||||||||||||||
| solubility |
easily in water (587 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Bis (hydroxylammonium) sulfate is a chemical compound from the group of hydroxylammonium salts and sulfates .
Extraction and presentation
Bis (hydroxylammonium) sulfate can be obtained by an acid-base reaction of hydroxylamine with sulfuric acid.
properties
Bis (hydroxylammonium) sulfate is a colorless or white, water-soluble and hygroscopic solid. It is unstable at elevated temperatures and its aqueous solution is acidic.
use
Bis (hydroxylammonium) sulfate is used as a reducing agent in photography and dyeing, depilatory for hides and for cleaning aldehydes and ketones .
safety instructions
Bis (hydroxylammonium) sulfate is an explosive solid. In the event of impact or friction, heating or other ignition sources, it reacts with rapid decomposition, producing sulfur oxides, nitrogen oxides, hydroxylamine, ammonia and nitrogen.
In Germany, hydroxylammonium sulfate is classified as an explosive substance in group C in accordance with the regulations of the Explosives Act.
Web links
- International Chemical Safety Card 0898
- Process for the preparation of hydroxylammonium sulfate (Freepatentsonline)
Individual evidence
- ↑ a b c d e f g h i Entry on bis (hydroxylammonium) sulphate in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Entry on bis (hydroxylammonium) sulphate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Toxicological assessment of hydroxylamine and its salts (PDF) from the professional association for raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
- ^ Announcement of the new findings made by BAM since 1987 in accordance with § 2 SprengG pdf-Link .





