Imidazoles

Imidazoles are a chemical group from the field of heterocyclic compounds and belong to the group of azoles . Its members are five-membered cyclic organic unsaturated compounds that have exactly two non-adjacent nitrogen atoms in the ring structure. They belong to the group of heteroaromatics and are isomeric to the pyrazoles . The parent system of the group is imidazole .
Occurrence
Imidazoles occur in many ways in nature. The amino acid histidine and the histamine derived from it contain an imidazole ring, as do the purines , xanthines and their derivatives. Imidazole alkaloids are found in the rhombus family .
presentation
The synthesis of imidazoles can be done starting from α, β-Di ketones and aldehydes . For this purpose, all carbonyl functions are first converted to imines with ammonium acetate (NH 4 OAc) . Acid catalyzed the coupling of a diimine nitrogen to the imine carbon with the formation of a diiminoamine. Also acid- catalyzed , the nucleophilic attack of the amine nitrogen on the second imine carbon now follows , with the ring being closed. The resulting amino function is then split off as ammonia , creating a diimine which then tautomerizes to form the imidazole .
properties
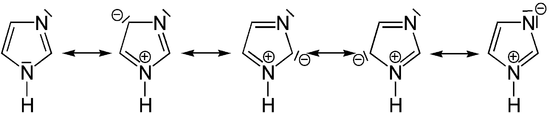
Imidazoles have six π electrons that form a mesomeric system. In addition to the two double bonds in the ring, the free electron pair of the amine nitrogen is also added. The lone pair of electrons in the imine nitrogen cannot take part in the mesomerism because it is located in the plane of the ring and, for geometric reasons, cannot overlap with the π system above and below the plane of the ring. The imidazole ring is planar, so that imidazoles meet the Hückel criteria and have an aromatic character.
Like all azoles, imidazoles have a basic character. The pKa value of protonated imidazole is 7.0. The relatively high basicity compared to pyrazoles is explained by the cyclic amidine structure . In addition, however, they can also occur as acids, whereby the nitrogen-bonded proton can be split off, so that they belong to the ampholytes .
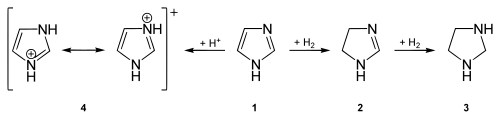
The partial hydrogenation of imidazoles creates imidazolines (see also imidazoline receptor ). If imidazoles are completely hydrogenated, imidazolidines are formed .
use
The imidazolium cation is easily formed from imidazole . Derivatives of this cation with various organic radicals are used as ionic liquids . Well-known derivatives are z. B. xylometazoline or clotrimazole .
Web links
Individual evidence
- ↑ a b c d D. T. Davies: Basistexte Chemie: Aromatic Heterocyclen , 1st edition, Wiley-VCH, Weinheim 1995, ISBN 3-527-29289-6 .
- ↑ a b H. Beyer, W. Walter: Textbook of Organic Chemistry , 23rd Edition, S. Hirzel Verlag, Stuttgart 1998, pp. 753-876, ISBN 3-7776-0808-4 .
- ↑ J. Falbe, M. Regitz (Ed.): Römpp Lexikon Chemie . 10th edition, Thieme, Stuttgart a. New York, 1996-1999. P. 1882.