Indolamine 2,3-dioxygenase
| Indolamine 2,3-dioxygenase | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | 403 amino acids | |
| Cofactor | Hamm | |
| Identifier | ||
| Gene names | INDO IDO; IDO-1; | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.13.11.52 , dioxygenase | |
| Response type | Oxidation with the incorporation of two oxygen atoms | |
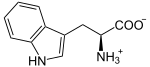
| Substrate | L -Tryptophan + O 2 | |
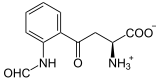
| Products | N -formylkynurenine | |
| Occurrence | ||
| Homology family | Indolamine 2,3-dioxygenase | |
| Parent taxon | Mushrooms, animals | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 3620 | 15930 |
| Ensemble | ENSG00000131203 | ENSMUSG00000031551 |
| UniProt | P14902 | P28776 |
| Refseq (mRNA) | NM_002164 | NM_001293690 |
| Refseq (protein) | NP_002155 | NP_001280619 |
| Gene locus | Chr 8: 39.9 - 39.93 Mb | Chr 8: 24.58 - 24.6 Mb |
| PubMed search | 3620 |
15930
|
Indolamine-2,3-Dioxygenase (IDO) is the enzyme that breaks down tryptophan to N-formylkynurenine. Unlike the tryptophan 2,3-dioxygenase (TDO) IDO is produced in all tissue types in the human body, but especially in the tonsils and the placenta , where the degradation of tryptophan one over the normal catabolism is beyond purpose: to support the immune system in Infections on the one hand, and prevention of fetal rejection on the other. Due to its immunosuppressive effect, it is a very promising target for achieving longer acceptance of transplants. Conversely, their inhibition could improve the fight against tumors.
The INDO - gene probably originated by a copy of the TDO2 gene.
Catalyzed reaction
L -ryptophan is oxidized to N -formyl- L -kynurenine. D- Triptophan is also accepted as a substrate . Also superoxide can act as oxygen donor.
Medical importance
Functions in the immune system
Both the immunosuppressive and immune-supporting function of IDO can be explained by the high value of the essential amino acid tryptophan, which is particularly required during the activation of T cells , but also by invading foreign cells. With the rapid breakdown of all tryptophans by IDO, protein synthesis is effectively paralyzed in the local area . In addition, the resulting degradation products activate the generation of regulatory T cells , which are ultimately responsible for immunosuppression .
Neurophysiology
Changes in tryptophan metabolism associated with indolamine-2,3-dioxygenase (IDO) and tryptophan-2,3-dioxygenase (TDO) are important for neuropsychiatric research. Changes in enzymatic activities along the tryptophan-kynurenine metabolic pathway have been described for numerous disorders. In particular, the IDO can change the activity of kynurenine formidase and kynurenine-3-monooxygenase . Typically, this leads to an accumulation of kynurenine and a shift in tryptophan metabolism towards kynurenic acid, anthranilic acid and their other metabolic products. Such changes are described for diseases of the brain (neurological and psychiatric diseases such as schizophrenia and tic disorders ) and the liver. A common constellation in various inflammatory (e.g. rheumatoid arthritis ), neuropsychiatric and malignant diseases is a simultaneously increased kynurenine / tryptophan ratio due to the accumulation of kynurenine before the next metabolic step, the hydroxylation to 3-hydroxykynurenine as a result of catalysis by kynurenine-3-monooxygenase (KMO).
Immune tolerance in lymph nodes
Lymph nodes that are downstream from a tumor are privileged, so to speak; A particularly large number of tumor antigens flow through them . It is important that the immune response does not overshoot in this area, as otherwise a lot of normal tissue would suffer. For this reason, the body increases the production of IDO in such lymph nodes in order to dampen the immune response. On the other hand, this can lead to IDO being increasingly produced in tumor tissue and then tumor antigens no longer being presented and therefore not recognized as foreign in the further course. In fact, IDO is overexpressed in several cancer cell lines. One tries, therefore, to achieve a better fight against cancer by inhibiting IDO.
The same mechanism is the cause when chronic infections facilitate local tumor growth, because here, too, the downstream lymph nodes below the focus of infection increasingly produce IDO and thus prevent a complete immune response from T cells . Mice with decreased IDO production did not show this phenomenon.
Immune tolerance in pregnancy
The original formulation of the paradox that the fetus is not rejected goes back to Medawar. Munn showed that inhibition of IDO with 1-methyl tryptophan to a rejection of the mouse model in 1998 Conceptus leads.
regulation
The production of IDO is stimulated by gamma interferon and lipopolysaccharides . The cancer-inhibiting effect of curcumin is at least partly due to the disruption of this signaling pathway and subsequent inhibition of IDO. The signaling pathway also appears to play a role in the establishment of an HIV infection.
1-L- methyltryptophan , the alkaloid exiguamin A and derivatives of menadione are known to be inhibitors of IDO .
Web links
- tryptophan + O2 => N-formylkynurenine [IDO] reactome.org
Individual evidence
- ↑ BioGPS entry
- ↑ OMIM entry
- ↑ Quan J, Tan PH, MacDonald A, Friend PJ: Manipulation of indoleamine 2,3-dioxygenase (IDO) for clinical transplantation: promises and challenges . In: Expert Opin Biol Ther . 8, No. 11, November 2008, pp. 1705-1719. doi : 10.1517 / 14712598.8.11.1705 . PMID 18847306 .
- ↑ Yuasa HJ, Takubo M, Takahashi A, Hasegawa T, Noma H, Suzuki T: Evolution of vertebrate indoleamine 2,3-dioxygenases . In: J. Mol. Evol. . 65, No. 6, December 2007, pp. 705-714. doi : 10.1007 / s00239-007-9049-1 . PMID 18026683 .
- ↑ UniProt P14902
- ↑ Werner ER, Werner-Felmayer G: Substrate and cofactor requirements of indoleamine 2,3-dioxygenase in interferon-gamma-treated cells: utilization of oxygen rather than superoxide . In: Curr. Drug metab. . 8, No. 3, April 2007, pp. 201-203. PMID 17430107 .
- ↑ Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR: The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation . In: J. Immunol. . 181, No. 8, October 2008, pp. 5396-5404. PMID 18832696 .
- ↑ AJ Dunn, AH Swiergiel, R. de Beaurepaire: Cytokines as mediators of depression: what can we learn from animal studies? In: Neurosci Biobehav Rev. , 2005, 29 (4-5), pp. 891-909.
- ↑ TJ Connor, N Starr, JB O'Sullivan, A. Harkin: Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma? In: Neurosci Lett. , 2008 Aug 15; 441 (1), pp. 29-34. doi: 10.1016 / j.neulet.2008.06.007 . Epub 2008 Jun 7.
- ↑ JM. Loftis: Indolamine 2,3-dioxygenase regulation and neuropsychiatric symptoms . In: Psychoneuroendocrinology . 2013 Sep; 38 (9), pp. 1829-1830. doi: 10.1016 / j.psyneuen.2013.05.020 . Epub 2013 Jun 27.
- ^ Norbert Müller: The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders - relation to drug treatment . In: Dialogues Clin Neurosci. 2009, 11, pp. 319-332.
- ↑ Robert Dantzer, Jason C. O'Connor, Gregory G. Freund et al .: From inflammation to sickness and depression: when the immune system subjugates the brain . Nature Publishing Group. January 2008, Volume 9.
- ↑ N Müller, AM Myint, MJ Schwarz: Inflammatory Biomarkers and Depression . In: Neurotox Res. , 19, 2010, pp. 308-318.
- ↑ Ikwunga Wonodi, O. Colin Stine, Korrapati V. Sathyasaikumar et al .: Downregulated Kynurenine 3-Monooxygenase Gene Expression and Enzyme Activity in Schizophrenia and Genetic Association With Schizophrenia Endophenotypes . In: Arch Gen Psychiatry , 2011, 68 (7), pp. 665-674
- ^ N Müller, D Krause, E Weidinger, M Schwarz: Immunological treatment options for schizophrenia . In: Fortschr Neurol Psychiatr. , 2014 Apr; 82 (4), pp. 210-219, doi: 10.1055 / s-0033-1355776 .
- ↑ Maria Holtze, Peter Saetre, Göran Engberg et al .: Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls . In: J Psychiatry Neurosci. , 2012; 37, pp. 53-57.
- ↑ Brian M. Campbell, Erik Charych, Anna W. Lee, Thomas Möller: Kynurenines in CNS disease: regulation by inflammatory cytokines. Frontiers in Neuroscience . In: Neuroendocrine Science , 2014, Volume 8, Article 12.
- ^ PJ Hoekstra, GM Anderson, PW Troost: Plasma kynurenine and related measures in tic disorder patients . In: Eur Child Adolesc Psychiatry , 2007 Jun, 16 Suppl 1, pp. 71-77.
- ↑ Serdar M. Dursun, Gillian Farrar, Sheila L. Handley et al .: Elevated plasma kynurenine in Tourette syndrome . Molecular and Chemical Neuropathology 1994, 21: pp. 55-60
- ↑ H. Rickards, SM Dursuna, G. Farrar: Increased plasma kynurenine and its relationship to neopterin and tryptophan in Tourette's syndrome . In: Psychological Medicine , 26, 1996, pp. 857-862
- ↑ Erik Kwidzinski: Involvement of indolamine 2,3-dioxygenase (IDO) in immune regulation of the central nervous system . Dissertation, Humboldt-Universität zu Berlin, Medical Faculty - Universitätsklinikum Charité, published on February 13, 2006, urn : nbn: de: kobv: 11-10059777
- ↑ A Buness, A Roth, A Herrmann, O Schmitz, H Kamp et al .: Identification of Metabolites, Clinical Chemistry Markers and Transcripts Associated with Hepatotoxicity . In: PLoS ONE 9, 2014, p. E97249. doi: 10.1371 / journal.pone.0097249
- ↑ Hirata Yukiko, Kawachi Takashi, Sugimura Takashi: Fatty liver induced by injection of L-tryptophan . In: Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 144, 1967, pp. 233-241.
- ↑ K. Schroecksnadel, S. Kaser, G. Neurauter et al .: Increased Degradation of Tryptophan in Blood of Patients with Rheumatoid Arthritis . In: The Journal of Rheumatology , 2003, 30, p. 9
- ↑ M Maes, R Verkerk, S Bonaccorso et al .: Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation . In: Life Sci . , 2002, 71: pp. 1837-1848.
- ↑ Katz JB, Muller AJ, Prendergast GC: Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape . In: Immunol. Rev. . 222, April 2008, pp. 206-221. doi : 10.1111 / j.1600-065X.2008.00610.x . PMID 18364004 .
- ↑ AJ Muller, MD Sharma, PR Chandler et al .: Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase . In: Proc. Natl. Acad. Sci. USA . 105, No. 44, November 2008, pp. 17073-17078. doi : 10.1073 / pnas.0806173105 . PMID 18952840 .
- ↑ Medawar, PB: Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 7, pp. 320-338, 1953.
- ^ Billington WD: The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar . In: J. Reprod. Immunol. . 60, No. 1, October 2003, pp. 1-11. PMID 14568673 .
- ^ DH Munn, M Zhou, JT Attwood et al .: Prevention of allogeneic fetal rejection by tryptophan catabolism . In: Science (journal) . 281, No. 5380, August 1998, pp. 1191-1193. PMID 9712583 .
- ↑ YI Jeong, SW Kim, ID Jung et al .: Curcumin suppresses the induction of indoleamine 2,3-dioxygenase by blocking the JAK-PKC-delta-STAT1 signaling pathway in IFN-gamma-stimulated murine dendritic cells . In: J. Biol. Chem. . December 2008. doi : 10.1074 / jbc.M807328200 . PMID 19075017 .
- ↑ Schroecksnadel K, Winkler C, Werner ER, et al : Interferon-gamma-mediated pathways and in vitro PBMC proliferation in HIV-infected patients . In: Biol. Chem. . November 2008. doi : 10.1515 / BC.2009.018 . PMID 19040353 .
- ↑ JC O'Connor, MA Lawson, C André et al .: Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice . In: Mol. Psychiatry . January 2008. doi : 10.1038 / sj.mp.4002148 . PMID 18195714 .
- ↑ Lob S, Konigsrainer A, Schafer R, Rammen HG, Opelz G, Terness P: Levobut not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells . In: Blood . 111, No. 4, February 2008, pp. 2152-2154. doi : 10.1182 / blood-2007-10-116111 . PMID 18045970 .
- ↑ HC Brastianos, E Vottero, BO Patrick et al .: Exiguamine A, an indoleamine-2,3-dioxygenase (IDO) inhibitor isolated from the marine sponge Neopetrosia exigua . In: J. Am. Chem. Soc. . 128, No. 50, December 2006, pp. 16046-16047. doi : 10.1021 / ja067211 + . PMID 17165752 .
- ↑ S Kumar, WP Malachowski, JB DuHadaway et al .: Indoleamine 2,3-dioxygenase is the anticancer target for a novel series of potent naphthoquinone-based inhibitors . In: J. Med. Chem. . 51, No. 6, March 2008, pp. 1706-1718. doi : 10.1021 / jm7014155 . PMID 18318466 .