Indophenol blue
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
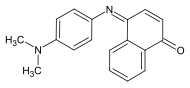
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Indophenol blue | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 18 H 16 N 2 O | ||||||||||||||||||
| Brief description |
dark blue powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 276.3 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
168-170 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Indophenol blue is a dye from the group of quinone imine dyes .
Manufacturing
In the first step in the production of indophenol blue, N , N- dimethyl-1,4-phenylenediamine is oxidized with iodine or sodium persulfate to give the quinonediimine . This intermediate reacts with 1-naphthol at the para position to the hydroxyl group in the sense of an electrophilic aromatic substitution . The resulting diarylamine is oxidized to indophenol in the last step:
use
The reaction of an aqueous solution of 1-naphthol and N , N- dimethyl-1,4-phenylenediamine is used as a NADI reagent for the biochemical detection of the enzyme cytochrome c oxidase ( oxidase test ) in bacteria and in histology .
Individual evidence
- ↑ a b data sheet Indophenol Blue from Sigma-Aldrich , accessed on July 23, 2019 ( PDF ).
- ↑ a b Entry on Indophenol Blue at TCI Europe, accessed on July 23, 2019.
- ↑ Ulfrich Nickel: Reactions with Wurster's cations . In: Chemistry in Our Time . tape 12 , no. 3 , 1978, p. 89-98 , doi : 10.1002 / ciuz.19780120305 .
- ↑ WL Gaby, C. Hadley: Practical laboratory test for the identification of Pseudomonas aeruginosa , in: J. Bacteriol. 1957, 74 , 356-358, PMID 13475249 , PMC 314647 (free full text).


