Isopyrazam
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
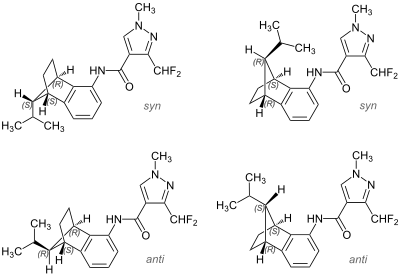
|
|||||||||
| Mixture of two syn isomers (top) and two anti isomers (bottom) | |||||||||
| General | |||||||||
| Surname | Isopyrazam | ||||||||
| other names |
3- (Difluoromethyl) -1-methyl- N - [1,2,3,4-tetrahydro-9- (1-methylethyl) -1,4-methanonaphthalen-5-yl] -1 H -pyrazole-4-carboxamide |
||||||||
| Molecular formula | C 20 H 23 F 2 N 3 O | ||||||||
| Brief description |
white odorless solid |
||||||||
| External identifiers / databases | |||||||||
|
|||||||||
| properties | |||||||||
| Molar mass | 359.41 g mol −1 | ||||||||
| Physical state |
firmly |
||||||||
| density |
1.332 g cm −3 |
||||||||
| Melting point |
130.2-144.5 ° C |
||||||||
| boiling point |
261-274 ° C |
||||||||
| solubility |
|
||||||||
| safety instructions | |||||||||
|
|||||||||
| Toxicological data | |||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Isopyrazam is a mixture of four isomeric chemical compounds from the group of pyrazoles and carboxamides .
properties
Isopyrazam is a white odorless powder that is practically insoluble in water. It's a mix of
- two syn - isomers 3- (difluoromethyl) -1-methyl- N - [(1 RS , 4 SR , 9 RS ) -1,2,3,4-tetrahydro-9-isopropyl-1,4-methanonaphthalene-5- yl] pyrazole-4-carboxamide and
- two anti -isomers 3- (difluoromethyl) -1-methyl- N - [(1 RS , 4 SR , 9 SR ) -1,2,3,4-tetrahydro-9-isopropyl-1,4-methanonaphthalene-5- yl] pyrazole-4-carboxamide.
The technical product contains 78–100% syn and a maximum of 15% of the anti isomers.
use
Isopyrazam is used as a fungicide . It was developed by Syngenta to combat the Black Sigatoka fungus in bananas and works by inhibiting succinate dehydrogenase . In Europe it is said to be used as a fungicide on grain.
Admission
The EU Commission approved the use of isopyrazam as an active ingredient in plant protection products with effect from April 1, 2013.
In Germany and Austria, plant protection products (e.g. Bontima) with this active ingredient are approved against various fungal diseases in grain , but none in Switzerland. Isopyrazam was first approved in England in 2010.
safety instructions
There is evidence that isopyrazam causes liver and uterine tumors in female rats.
Individual evidence
- ↑ a b c d e f g h i j k l m data sheet isopyrazam from Sigma-Aldrich , accessed on May 22, 2017 ( PDF ).
- ↑ Alan Wood: Isopyrazam
- ↑ a b EFSA: Conclusion on the peer review of the pesticide risk assessment of the active substance isopyrazam. In: EFSA Journal. 10, 2012, doi : 10.2903 / j.efsa.2012.2600 .
- ↑ EPA: Pesticid Fact Sheet Isopyrazam (PDF; 6.7 MB), October 5, 2011.
- ↑ Implementing Regulation (EU) No. 1037/2012 of the Commission of November 7, 2012 on the approval of the active substance isopyrazam (PDF) in accordance with Regulation (EC) No. 1107/2009 of the European Parliament and of the Council on the placing of plant protection products on the market and amending it of the Annex of the Commission Implementing Regulation (EU) No. 540/2011.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on isopyrazam in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; Retrieved February 20, 2016.
- ↑ Chemie.de: Syngenta receives first approval for isopyrazam plant protection product in Europe , April 1, 2010.


