Artificial pancreas
An artificial pancreas , artificial pancreas or artificial beta cell is a medical device that supplies patients with diabetes mellitus with insulin as a function of continuous measurements of the blood sugar level . It simulates the functioning of the beta cells in the islets of Langerhans in the pancreas , which produce and release insulin in the body and which in diabetics are destroyed or their function is restricted. The function of an artificial pancreas corresponds more closely to the natural release of insulin than conventional insulin therapy or treatment with the help of an insulin pump .
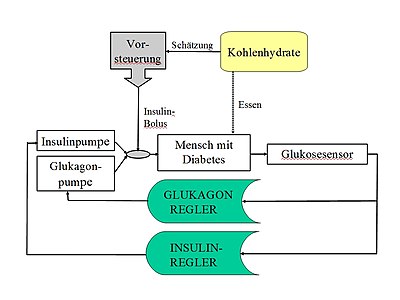
The essential components of an artificial pancreas are a continuously measuring glucose sensor for determining blood sugar, a pump for controlled insulin delivery and a miniaturized computer that evaluates the measurement data from the sensor and controls the pump using an algorithm to simulate the glucose-insulin control circuit. The artificial pancreas has been in development since around 1970, and corresponding systems were first tested on humans from the late 1970s. The aim is long-term use as an implant or as a device that can be worn by the patient. At the moment, the artificial pancreas is still the subject of research and, apart from devices for short-term inpatient use, is not yet commercially available.
history
Kadish first described a control loop in 1964 . From 1974 onwards, several research groups around the world tried to get closer to the goal of a regulated insulin infusion, including Albisser (USA), Kreagen (Australia), Mirouze (France) and Shishiri (Japan). In Germany, EF Pfeiffer († January 23, 1997) in Ulm worked on the development. In Karlsburg in the GDR, U. Fischer and colleagues were simultaneously researching a system with a mathematical model and a glucose sensor. At the same time in Japan, M. Shishiri brought out a subcutaneous measuring device. However, neither of them came into the clinical development of miniaturized devices. Large devices with non-implanted sensors and glucose counter-infusion were brought into commercial production during this time ( Biostator and Nikkiso STG-22 Blood Glucose Controller). From 1978 the first subcutaneous insulin pumps were operated by Pickup in England and Tamborlane in the USA. It was only after reliable and implantable continuous glucose sensors could be produced in large numbers from 2004 onwards, primarily in the USA, that numerous development priorities came back to focus (table). The approval of a simulator by the US Food and Drug Administration FDA as a substitute for animal experiments in 2008 made it easier to simulate experiments with the control loops ( "in silico" ), which triggered a new boost in development. From 2011, the full portability of the control could be achieved by transferring the complete software to Android smartphones (DIAS system).
Subgroups, types
There are different versions, which differ by
- Choice of the compartment for the position of the sensor and the insulin catheter, subcutaneous, intravenous or intraperitoneal
- Degree of automation
- Execution as miniaturization, fully implanted, external large device
- Place of application: intensive care unit, inpatient, outpatient
- Target range or exact target value
- Insulin alone or bi-hormonal with glucagon or pramlintide
- Type of control algorithm, heart rate
The most important are discussed in detail below
Threshold interrupt system
The aim of a threshold suspend device system for insulin is to reduce the severity or reverse a dangerous drop in blood glucose (hypoglycemia) by temporarily interrupting the insulin supply by means of a pump when the glucose level reaches a threshold value or is close to it approximates.
Systems that predict the reaching of the threshold are a further development (predictive low-glucose suspend PLGS). In a study from the USA with a predictive switch-off, 78% of the blood glucose values were between 70 and 200 mg / dl in the nights with PLGS compared to 71% without such a predictive switch-off.
Area control systems
A control-to-range (CTR) system reduces the likelihood of hyper- or hypoglycaemia by changing the insulin dose as blood glucose approaches a high or low threshold. People who choose this type of system must continue to self-inject insulin, perform blood glucose self-monitoring and adjust the insulin dose to these values.
Setpoint control systems
Setpoint control systems (Control-to-target, CTT) try to achieve this setpoint or target value at any time. The system works fully automatically and does not require any user involvement other than calibrations.
Bi-hormonal regulation system

A bi-hormonal control system should achieve a glucose target value using two algorithms, each of which controls a pump for a lowering hormone ( insulin ) and a lifting hormone ( glucagon ). This type of regulation mimics the glucose regulation of a healthy organism better. Glucagon is formed in the α cells of the pancreas in healthy individuals and has so far been approved as a drug for parenteral use in severe hypoglycaemia. Disadvantages: The long-term safety of glucagon has not yet been tested. A stable formulation is difficult because of the formation of fibrils and aggregates in aqueous solution and the degradation. Advantage: Glucagon has a faster flooding time than insulin analogues (approx. 20 min to the maximum.)
Hybrid system with pilot control
A hybrid system allows the patient to administer an additional dose of insulin before a meal. This additional dose reduces the risk of hyperglycaemia after the meal. The usual in control engineering method of the feedforward control (engl. Foreward feed) for compensation of measurable disturbance variables is partially implemented here. Since the disturbance cannot be recorded exactly, but can only be estimated by the user (carbohydrate or KE estimation), i. d. Usually operated a partial pilot control, in which z. B. 50% of the calculated insulin dose can be injected in advance as a bolus. The principle of fully automatic control is thus abandoned in favor of better regulation of meals and the help of the user is requested.
Controller types
Model predictive controller
Model predictive control (MPC) are used in technology in refineries, waste incineration, etc. when common controllers (PID) do not have the necessary quality and there is enough time to optimize the control with each sampling step. You can recalculate the control parameters based on a prediction after each sampling.
Proportional-integral-differential controller (PID)
This type of controller consists of three components: (a) the proportional part in which the manipulated variable (infusion rate) is proportional to the control difference (actual glucose value - nominal glucose value). (b) An integrator, which ensures the steady-state accuracy, but slows down the response behavior. (c) a differentiator that reacts to changes, makes the controller fast, but amplifies rapid disturbances and can make the controller unstable. From 2010, such regulators - based on the physiological regulation of a healthy pancreas - received feedback from the (predicted) insulin level (IOB) in order to prevent hypoglycaemia caused by overinsulinization.
In 2017, with the Minimed 670G, a system came onto the market in the USA alone, in which the basal rate is regulated using a PID algorithm with IOB feedback. This system does not record meals, so it can be classified as a hybrid system (see above).
Fuzzy controller
Fuzzy control consists of a controller, which a number of discrete input values (e.g. three glucose ranges: high, normal, low) by fuzzy rules ('if' - 'then' rules) an output value (insulin infusion rate ) result. Here, approximate values for the insulin doses are generated from empirical knowledge, which is close to the dose recommendations of diabetologists. Thus, the fuzzy control is based on the common practice of bolus calculation in everyday life: the patient corrects between 140 and 170 mg / dl with one insulin unit (iU) and between 170 and 200 mg / dl with 2 iU etc.
Risk reduction and security architecture
An incorrect measurement by the sensor and / or an incorrect control by the control algorithm can in principle lead to life-threatening hypoglycemia . A modular structure in which safety modules can output an insulin switch-off or warning independently of the control algorithm is required. So z. B. a technical limitation of the infusion rate provided by a separate hypoglycemia prediction algorithm (English. Low glucose detection module) or by an and insulin-on-board calculation.
Peculiarities and difficulties
A special feature of the feedback are long time delays in the process: a physiological and therefore unaffected delay in action is the action of insulin in the liver of around 100 minutes and in the peripheral tissue (muscles) of around 20 minutes. In addition, with the currently preferred sc-sc application, there are potentially changeable delays at both ends: the lag time of about 5–15 minutes due to the glucose sensor and tissue diffusion and the delay resulting from insulin absorption . With fast-acting insulin analogues, the maximum values in the blood plasma are reached after 0.5–2 hours, the duration of action is 3–5 hours. Classic controller types (PID) can partially compensate for such delays by amplifying the D element, but this is at the expense of the amplification of the sensor noise and other disturbances. Modern controller types (MPC) can record such delays better, but they also reach their limits here. Solutions for other forms of insulin administration ( intraperitoneal , by inhalation ) are currently being sought intensively.
The individualization of algorithms is increasingly understood. If disturbances (meals, sports) are deliberately run through such a system, adjustments to the parameters can be determined more effectively and, under favorable circumstances, adjusted in real time.
Performance goals and metrics
- Complete or extensive transfer of all diabetes management activities from the patient to the device (relief)
- Stabilization and reduction of the mean glycaemia with long-term reduction of the consequential damage
- Avoidance or reduction of acute complications such as hypoglycaemia and ketoacidotic comas,
It is proposed to measure the quality and variability by means of time in target (TIR), which is possible with continuous glucose measurement (CGM). Hybrid systems (see above) currently achieve that the glucose values are in the range 70–180 mg / dl in around 70% of the measurement time. In addition, it makes sense to record the time in the hypoglycemic range. Furthermore, the measurement of quality of life will play an increasingly important role. There are validated instruments to measure these e.g. B. Entry forms for Diabetes Quality of Life or Fear of Hypoglycemia .
Ultimately, a compromise must always be made between the degree of automation and the controller quality; just such a compromise between achieving euglycemia and the number of undesirable hypoglycemias. The partial transfer to the user also harbors dangers that are based on unpredictable human behavior and raise safety concerns.
Comparative metrics are produced by national registers or quality initiatives (“non-inferiority” due to the best previous comparative therapy). In Germany, for example, a combined therapy and training program initiated in a hospital with the methods of quality management showed that people with type 1 diabetes can achieve an HbA1c of 7.3% on average with intensive insulin therapy (ICT ) and an HbA1c- independent number of severe hypoglycaemia of 0.14 / patient / year.
Research focus worldwide
| AP system | algorithm | developer | Duration of the scheme |
Meals (pre-control) |
Sport incl. | outside of the house | Pump sensor |
|---|---|---|---|---|---|---|---|
| PID controller (USA) | PI / PD-IFB | G.Steil | 14 hours | n | n | n | Animas Pump, Abbott Free Style Navigator |
| MD-Logic (Isr / D / Slo) | Fuzzy | E. Atlas | N / A | N / A | j | j | Enlite; Veo pump (Medtronic) |
| DIAS (USA / It / Fr) | MPC | Kovatchev / Cobelli / Renard / Zisser |
40 hours | j | n | j | Tandem pump, DexCom G4 |
| Florence (En / USA) | MPC | R.Hovorka | 8 hours | j | n | n | Dana R Diabecare; Abbott Free Style Nav |
| Bionic Dual Hormone (USA) |
Insulin and glucagon adapt. MPC |
Damiano | 120 hours | j | j | j | Tandem t: slim; Dexcom G4 |
| AP @ home (EU) | MPC | Multiple authors | N / A | j | N / A | j | Omnipod pump; Dexcom seven + |
| 12 Week 24/7 (USA / It) | MPC zone | multiple authors | 12 weeks | j | n | j | Roche AccuCheck Combo Spirit, Dexcom G4 |
| Tandem with TypeZero Tech, (USA) | Control-IQ Technologies | iDCL Trial Research Group | 6 months | j | n | j | t: slim X2 insulin pump (tandem) with Control-IQ, (TypeZero), Dexcom G6 sensor |
Notes: not complete selection, a complete selection can be found in the Articial Pancreas Clinical Trial Database; MPC = model predictive control, IFB = insulin feedback, J = fulfilled N = not fulfilled N / A = unknown;
State of the art
So-called sc-sc systems are currently being tested, in which the glucose sensor is located in the subcutaneous tissue (SC) as is the infusion catheter for insulin. Even if there would be no significant delays in an intravenous measurement or infusion, the route is currently via the subcutaneous compartment for safety reasons. Modern systems can be designed in such a way that wireless connections ( Bluetooth ) and the complete software for control can be installed on a mobile device (smart phone). In principle, remote monitoring ( telemonitoring ) is also possible.
Two systems have received a CE mark , one of which is available in European countries and is listed in the German list of medical aids used by the statutory health insurance . Another 16 systems have been identified that have commercial intentions.
The studies with real patients aim to extend the applications to ever longer periods and to allow the users more independence. Initially, supervised diabetes camps and later hotels close to clinics were selected. In a study from England, for example, safe domestic use for up to three months and an increase in the above-mentioned values were found. Target range described by 11%. In a multi-center study (mainly in the USA) in 2017, a system was tested on an outpatient basis in 29 adults over 12 weeks, which included a pre-control of meals. New here was the weekly adaptation of the basal rate and the insulin-to-carbohydrate ratio (which determines the so-called meal bolus) on remote servers. As a result, the HbA1c could be reduced from originally 7% to 6.7%. A similar increase in TIR was achieved in a multicenter study from 2019 on 112 patients over 6 months.
Non-commercial systems
OpenAPS has existed since 2013 under the motto “We are not waiting” , a worldwide do-it-yourself community that links commercial technologies to an artificial pancreas using algorithms and software in the sense of open source . The founders include Dana Lewis and Scott Leibrand from the USA.
As with commercial systems, OpenAPS or the somewhat newer AndroidAPS are hybrid systems, i. H. An insulin bolus must be delivered before each meal. In contrast to commercial systems that are subject to regulatory requirements (USA: FDA, Europe CE certificate for medical devices), such systems operate in a legal gray area. In order to avoid legal problems, the user has to assemble his product himself and he is not provided with any services. There is no liability for economic or health damage as a result of use.
Building it yourself requires technical skill, and membership in a social network such as Facebook is also required in order to exchange ideas with other “loopers”. The hardware required is a commercial insulin pump, a small computer (e.g. Intel Edison or Raspberry Pi ) and a CGM system that releases its raw data. The community expects user data to be donated anonymously. In 2018, between 500 and 1000 users were registered with openAPS.
literature
- Frederick Chee, Tyrone Fernando: Closed-Loop Control of Blood Glucose. Series: Lecture Notes in Control and Information Sciences. Volume 368.Springer, Berlin and New York 2007, ISBN 978-3-540-74030-8
- C.Cobelli, E. Renard, B.Kovatchev: Artificial Pancreas: Past, Present, Future in DIABETES 60: 2672 (2011) doi : 10.2337 / db11-0654
- Masami Hoshino, Yoshikura Haraguchi, Iwanori Mizushima, Motohiro Sakai: Recent Progress in Mechanical Artificial Pancreas. In: Journal of Artificial Organs. 12 (3) / 2009. Springer, pp. 141-149, ISSN 1434-7229
- Kavita Kumareswaran, Mark L Evans, Roman Hovorka: Artificial Pancreas: An Emerging Approach to Treat Type 1 Diabetes. In: Expert Review of Medical Devices. 6 (4) / 2009. Expert Reviews Ltd., pp. 401-410, ISSN 1743-4440
- Martina Lenzen-Schulte: Type 1 diabetes: DIY diabetes therapy . In: Deutsches Ärzteblatt . tape 116 , no. 29-30 , 2019, pp. A 1378-A 1381 ( aerzteblatt.de ).
Individual evidence
- ↑ Kadish AH. Automation control of blood sugar. I. A servomechanism for glucose monitoring and control. Am J Med Electron 1964; 3: 82-86
- ^ Pfeiffer EF, Thum C., Clemens AH The artificial Beta-Cell: A continuous control of blood sugar by external regulation of insulin infusion in Horm. Metabol. Res. 6 pp. 339-342 (1974).
- ↑ Fischer U. et al. Does physiological blood glucose control require an adaptive control strategy? IEEE Trans Biomed Eng. ; 34: 575-82. (1987).
- ↑ RM Bergenstal, DC Klonoff, SK Garg, BW Bode, M. Meredith, RH Slover, AJ Ahmann, JB Welsh, SW Lee, FR Kaufman: Threshold-based insulin-pump interruption for reduction of hypoglycemia. In: The New England Journal of Medicine . Volume 369, Number 3, July 2013, pp. 224-232, doi : 10.1056 / NEJMoa1303576 , PMID 23789889 .
- ↑ Spaic, T et al Predictive Hyperglycemia and Hypoglycemia Minimization: In-Home Evaluation of Safety, Feasibility, and Efficacy in Overnight Glucose Control in Type 1 Diabetes. Diabetes Care. 2017 40 (3): 359-366. doi : 10.2337 / dc16-1794 .
- ↑ Richalet J, et al. Model predictive heuristic control: Applications to industrial processes. Automatica.; 14: 413-28. (1978).
- ↑ A. Samann et al. Diabetologia (2005) 48: 1965-1970
- ↑ Dauber, Steil Diabetes Care 36: 222-227, 2013.
- ↑ Philip et al .: N Engl. J. med 368; 9: 824 (2013).
- ↑ Kovatchev et al. Diabetes Care 2014; 37: 1789.
- ↑ Hovorka et al. Diabetes Care 2014; 37: 1204-1211.
- ↑ J. Russell et al. N.Eng.J.Med (2014) doi : 10.1056 / NEJMoa1314474 .
- ↑ Luif et al. Diabetes Care. 2013; 36: 3882.
- ↑ a b Dassau et.al. 12-Week 24/7 Ambulatory Artificial Pancreas With Weekly Adaptation of Insulin Delivery Settings, Diabetes Care 2017; 46; P. 1719.
- ↑ a b Brown et al. NEJM 2019 Oct doi: 10.1056 / NEJMoa1907863
- ↑ Detailed overview of global research areas: Articial Pancreas Clinical Trial Database. The Doyle Group, accessed June 15, 2019 .
- ↑ S. Trevitt et al. Artificial Pancreas Device Systems for the Closed-Loop Control of Type 1 Diabetes: What Systems are in Development? Diab. Sci. Technol. November 2015.
- ↑ Thabit H, Tauschmann M, Allen JM et al. (2015) Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 373: 2129-2140-
- ↑ openaps.org
- ^ Dana Lewis, Scott Leibrand, #OpenAPS Community: Real-World Use of Open Source Artificial Pancreas Systems . In: Journal of Diabetes Science and Technology . tape 10 , no. 6 , November 2016, ISSN 1932-2968 , p. 1411-1411 , doi : 10.1177 / 1932296816665635 , PMID 27510442 ( sagepub.com [accessed May 14, 2019]).
- ↑ a b Stefanie Blockus: The compiled pancreas. Type 1 diabetics make artificial pancreases . In: c't . No. 9 , 2019, pp. 162 .
- ↑ Martina Lenzen-Schulte: Type 1 diabetes: diabetes therapy made by yourself . In: Deutsches Ärzteblatt . tape 116 , no. 29-30 , 2019, pp. A 1378-A 1381 ( aerzteblatt.de ).