Continuously measuring glucose sensor
Continuously measuring glucose sensors measure the tissue glucose concentration in the body. The Continuous Glucose Monitoring ( ger .: Continuous glucose monitoring CGM) is usually in people with diabetes used to be able to better control the therapy. On the one hand, you can show the glucose together with the temporal gradient (trend) on a display and save all values, which is currently used in commercial systems with needle sensors. They are also an integral part of a possible future, permanently functioning artificial pancreas , namely an insulin pump controlled by the blood sugar via an algorithm . Such a control circuit then basically consists of a continuously measuring glucose sensor and a controller that controls an insulin pump.
History and Development
Historically, such an enzyme sensor was designed as early as the 1960s. a. Biostator or Glucostator called, proved in the seventies the principle feasibility of a control circuit for the blood sugar regulation by means of continuous insulin infusion. Still, it took another twenty years for the first commercial sensor to hit the market.
Invasively applied glucose sensors
Invasive sensors have to be inserted through the intact skin, so the application is not entirely painless.
Needle sensors
The longest existing type is the traditional amperometric enzyme sensor with immobilized glucose oxidase (figure), it is realized in several of the types available on the market today. Glucose is measured selectively by the enzymatic conversion of glucose by the enzyme glucose oxidase (GOD), which is immobilized in a polymer (e.g. polyacrylamide) in the sensor tip or cross-linked with glutaraldehyde. In an electrochemical reaction, either the decrease in oxygen (O 2 ) or the formation of hydrogen peroxide H 2 O 2 is measured. So there will be an oxidation (producing electrons) on the working electrode or a reduction (consuming electrons) on the counter electrode. The advantage of the peroxide electrode is the simple structure in mass production, which is offset by the disadvantage that H 2 O 2 has a toxic effect on the GOD as well as on the electrode surface. This leads to stronger drifts and shortened lifetimes of these sensors. Shichiri and colleagues connected one of the first needle sensors to a portable artificial pancreas in Japan in 1983.
Technical specifications in vitro : The measuring range for glucose is typically 20–400 mg / dl, the accuracy is lower for low glucose values than for high values. The sensitivity of tiny needle sensors is typically 2 nA / mM (glucose concentration), there is always a background current. The response time (time to reach 63% of the equilibrium current with step function) is 1 to 3 minutes in vitro. The signal-to-noise ratio is between 3 and 10. Because of this noise, filters are necessary which add another time constant to the signal chain. A drift is based on the oxidative effect of H 2 O 2 , which not only destroys the glucose oxidase, but also the electrode surface and the membranes (biofouling). Therefore, the drift and the service life of a sensor in vitro are strongly dependent on the initial enzyme load and also on the methodology of how sensitive surfaces are protected from peroxide.
Specifications after implantation ( in vivo ): Since there are two substrates, namely O 2 and glucose, glucose should be the limiting substrate. In the body, however, the opposite concentration ratios exist with approx. 0.2 mM O 2 and 5.5 mM glucose. Advanced polymers as diffusion barriers come closer to this problem, as do electron mediators such as ferrocene. In vivo, the response time, service life and drift of amperometric needle sensors are particularly different from in vitro conditions. Diffusion processes between blood and tissue induce a delay time for the glucose concentration, which is specified between 3 and 10 minutes depending on the measurement method. As a rule, the implantation of the needle sensor leads to a tissue reaction. This adds an additional in-vivo component to the in-vitro drift and service life of the sensor . The trauma results in an inflammatory reaction, followed by a wound healing reaction with the accumulation of proteins with the subsequent formation of connective tissue with changing permeability for glucose and oxygen. Measures to master this are biocompatible surfaces, a limitation of the application duration and an interruption of the measuring process during the first hours of the "wound healing" after implantation .
Microdialysis
External sensors may be found. a. in microdialysis systems. Here, fluid is continuously pumped through a loop of semipermeable membranes located in the subcutaneous subcutaneous fatty tissue at flow rates of 2.5–10.0 µL / min. This liquid is enriched with the glucose there, depending on the concentration. Such a system was in the "Ulmer Zuckeruhr" presented by EF Pfeiffer († January 23, 1997) in Ulm in 1994. It consisted of a portable microdialysis system with an external glucose sensor and a telemetry device . It sent a signal to the receiver in the form of a wrist watch once a minute. Optical and acoustic alarms could be sent out in the event of high and low blood sugar levels. However, it was never really ready for the market, as the weight and dimensions, which corresponded to an old Walkman , were not suitable for everyday use. The Glucoday system from Menarini , which is commercially available today, also works according to this principle.
Advantages and disadvantages microdialysis systems: micro- dialysis systems operate at atmospheric pressure, thus have no oxygen deficit problem as needle sensors where the sensor is located in the body. Therefore, their measuring range is larger and their accuracy is higher than with needle sensors. A disadvantage is a dead time that inevitably arises from the transport of the dialysate and is in the range of minutes. A technical disadvantage can lie in the size and susceptibility of the pumping system to failure, as there are moving components.
fluorescence

The glucose measurement can also take place optically with the aid of fluorescence . Here a molecule is excited by an irradiated photon and a photon of lower energy is immediately emitted again. By using optical filters to separate the incident light and the emitted light (shifted to the reddish color), very selective measurements can be carried out.
However, there are no known molecules in the body that fluoresce in connection with glucose, so that foreign material from the outside must be brought into connection with the glucose-containing body fluid, similar to an electrochemical sensor (invasive measurement). Glucose-dependent emissions of light can be generated via a boric acid or concanavalin A -based glucose receptor and a quencher (figure). The method is sufficiently sensitive even in the low (hypoglycemic) range. In one application (from Glumetrics, USA), visible light from an LED is guided into an intravenous catheter via a glass fiber bundle the thickness of a wire and the red-shifted glucose-dependent light is returned to a sensor via the same path . Such a system can find use in intensive care medicine for intravenous monitoring. Another application (from Senseonics, USA) uses a 3 mm × 16 mm capsule which is brought into contact with the intercellular fluid under the skin . This capsule contains the fluorophores and wirelessly sends signals to a transmitter . A study on twelve diabetics over 90 days showed a MARD of 11.7% and a delay time of 7 minutes. This system was launched on the market at the 52nd Congress of the German Diabetes Society .
Non-invasive glucose sensors
Optical and dielectric sensors
Non-invasive sensors leave the skin and mucous membranes intact during the measurement and use electromagnetic waves for the measurement. So far, light in the near infrared range (measurement by absorption), but also in other frequency ranges (measurement by polarimetry or scattering ) have proven to be possibly suitable . With impedance spectroscopy at 20–60 MHz, the Swiss company Biovotion AG (formerly Solianis) introduced a completely new measurement method for glucose monitoring. When using several frequencies with this method, a self-calibrating sensor and a multiple network of artificial intelligence to learn the non-linear correlation function "blood sugar to impedance", a non-invasive blood sugar measuring system was developed by the company TROUT in Kassel. This system is patented; the functions were verified by means of a clinical test (user observation). So far, no sensor of this type has reached market maturity, not even for single measurements. Problems are the accuracy, the selectivity for glucose and the susceptibility to interference. Scientific studies on this are completely lacking. In so-called minimally invasive methods, for example, the skin is perforated almost painlessly with tiny holes (lasers) in order to gain tissue water. Of the optical methods, Raman spectroscopy showed the prospects of success for a number of years, as the glucose in the mid-infrared range between 500 and 900 nm leaves a clear spectral “fingerprint” that is concentration-dependent. The photoacoustic spectroscopy in the middle infrared region by means of quantum cascade laser shows encouraging results here on the bench, but still not a commercial system. Modern lasers and selective spectrographs as well as the possibility of miniaturizing complex arithmetic operations of the mathematical normalization and calibration process make a completely non-invasive form of a glucose sensor appear possible in the future.
GlucoWatch biographer
GlucoWatch Biographer (Animas Corporation, West Chester, PA , USA formerly Cygnus) was a real-time sensor that was withdrawn from the market in July 2007. It was the size of a wristwatch and was based on the measuring principle of "reverse iontophoresis ". Here, a direct current was periodically passed through the skin under the device, extracting tissue fluid from the tissue and osmotically taking tissue glucose with it. During the enzymatic measurement process on the "disposable electrodes" (usable for 13 hours, one measured value every 10 minutes) there was no electrically induced fluid exchange. As a side effect, moderate skin irritation and redness occurred, which limited the gestation period in practice and during scientific studies and were important for patient compliance . Two studies showed no improvement in metabolic management and therefore insurance coverage became unlikely (Miter study, DirecNet study). This presumably led to its withdrawal from the market.
Use in people with diabetes and effectiveness
Availability of values
- Permanent availability (push principle) of the value in the display. The permanent evaluation (signal processing) thus allows (1) to issue alarms for falling below or exceeding a predetermined threshold and (2) to make predictions of the glucose course.
- Demand-oriented display (pull principle), which takes place either by downloading the values to a computer or by sweeping over (scanning) and reading the glucose value and the trend on a separate device with display . The latter is implemented in a system (see table) that does not require any calibration by the user.
- The retrospective evaluation of the glucose course (possible with both principles) after the download to a PC (ideally with the injections and carbohydrate quantities) z. B. when meeting with a specialist is called professional continuous glucose monitoring (PCGM). It is z. T. reimbursed by health insurance companies.
Commercial systems
| company | Surname | indwelling life |
calibration | Measuring frequency |
MARD% | principle | annotation | |
|---|---|---|---|---|---|---|---|---|
| - | Abbott | FreeStyle Navigator II | 5 days | after 1, 2, 10, 24 and 72 hours | 1 min | 12.3% | Wired enzyme technology, needle sensor | Trend arrows, hypo-prediction statistics already available in the receiver Discontinued by the manufacturer as of December 31, 2017; Remaining stocks of consumables will be sold off. |
| + | Abbott | FreeStyle Libre | 14 days | no | 1 min | 11.4% | Wired enzyme technology, needle sensor | Read out glucose values by scanning with the receiver or smartphone (flash glucose monitoring: FGM), no alarms |
| + | Abbott | FreeStyle Libre 2 | 14 days | no | 1 min | 9.5% | Wired enzyme technology, needle sensor | Read out glucose values by scanning with the receiver or smartphone (flash glucose monitoring: FGM), alarms for hypoglycemia and hyperglycemia can be activated separately, each with an adjustable threshold |
| + | Dexcom | G4 Platinum | 7 days | every 12 hours | 5 min | 10.8% | Enzyme sensor | Hypo-alarm and trend arrows, combination with Animas Vibe insulin pump possible |
| + | Dexcom | G5 Mobile | 7 days | every 12 hours | 5 min | 9% * | Enzyme sensor | Smartphone compatible (wireless via Bluetooth Low Energy), allows use without parallel measurement |
| Dexcom | G6 Mobile | 10 days | no | 5 min | Enzyme sensor | |||
| + | Senseonics | Eversense XL CGM system | 180 days | 2 times a day | 5 min | 9.4% | fluorescence | Display of the data via smartphone app, various alarm functions also tactile directly via the HWZ transmitter, service life 149 days, sold in Europe by Roche Diabetes Care and Rubin Medical, insertion in specially trained diabetes centers |
| + | Medtronic | Enlite sensor | 6 days | every 12 hours | 5 min | 13.6% | Needle sensor electrochem. GOD | multiple alarm function
can be integrated in the pump |
| + | Medtronic | Guardian Sensor 3 | 7 days | every 12 hours | 5 min | 10.6% abdomen
9.1% (poor) |
Needle sensor electrochem. GOD | multiple alarm function
can be integrated in the pump |
| (+) | Menarini | Glucoday | 48 hours | 2 × in 48 hours | 10 min | n / A | Microdialysis | Must through med. Specialized personnel can be created |
| - | Animas | GlucoWatch biographer | 13 hours | 2 × in 48 hours | 10 min | n / A | Enzyme sensor, inverse iontophoresis | no longer manufactured |
Notes:
+ currently available, possibly also newer models
(+) available to a limited extent
- currently not available
MARD (mean absolute relative difference) = inverse measure of accuracy (the smaller the value, the better the system, information in%) is based on head-to-head comparisons and
* 10% for children from 2 years
Evaluation and approval of commercial systems
In the United States, it is approved for market launch by the Food and Drug Administration (FDA). In the European Union , medical devices are generally not approved, but are placed on the market . In 2018, the European Agency for Health Technology Assessment (HTA) EUnetHTA, under the leadership of the Ludwig-Boltzmann-Institut (Austria), issued an assessment of CGM and FGM based on current studies, which does not contain any explicit recommendations. Special approvals, e.g. B. by the Federal Institute for Drugs and Medical Devices , are possible in individual cases. A CE marking is required for placing on the market in the European Union, which may be affixed if the products meet the basic requirements. In most cases this is checked by a notified body . Germany: The evaluation, which leads to the assumption of costs according to § 35 SGB V by statutory health insurance companies, is carried out by the Federal Joint Committee (G-BA). This uses a benefit assessment by the Institute for Quality and Efficiency in Health Care (IQWIG), which published a report in 2015 based on the analysis of randomized studies on commercial real-time CGM systems. The assumption of costs by the health insurance companies was based on the G-BA decision below on individual decisions, which are based on reports from the Medical Service of the Health Insurance Funds (MDK). On June 16, 2016, the G-BA awarded the rtCGM (continuous interstitial glucose measurement with real-time measuring devices) in Germany as a statutory medical service for children and adults at the expense of the health insurance companies. The prerequisites for this blood sugar measurement, which was stipulated as a service by the statutory health insurance funds on September 7, 2016 a .:
- Insulin -dependent diabetes with intensified insulin therapy or insulin pump therapy and a completed CGM training course
- Prescription by a specialist in endocrinology / diabetology
- an individual therapy goal must be agreed with the patient and documented (during the course)
- the device used must be certified as a medical product
- At the request of the user, the data must be available to the treating physicians without third-party access (in particular the manufacturer) (data protection)
- Flash Glucose Monitoring (FGM) is excluded from this decision as it does not allow continuous tissue sugar measurement and therefore does not have alarm functions
Switzerland: In Switzerland, continuous glucose measurement (CGM) has been on the list of mediums and items (MiGeL) of the Federal Office of Public Health since 2016 and FGM (Freestyle Libre, Abbott) since July 2017. According to position 21.05 of the MiGeL, the CGM and the FGM are financed by the health insurance company under the following conditions:
- HbA1c value equal to or higher than 8% and / or in severe grade II or III hypoglycaemia or in severe forms of Brittle diabetes with an emergency consultation and / or hospitalization
- Prescribed only by endocrinology / diabetology specialists trained in the use of CGM technology.
- In the case of a period of use of more than six months, a prior confirmation of costs from the insurer is required based on a medical justification.
The guidelines of individual countries also regulate further details.
Testing on humans without an artificial pancreas

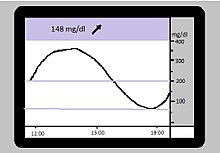
In this case, the sensor measurement was able to show retrospectively that the patient waited too long after the injections, especially with high blood sugar levels, and that this could lead to dangerous "crashes", e.g. T. induced into the hypoglycemic range. This escaped the individual measurements. The blue dashed lines correspond to the alarm limits (70 mg / dl and 180 mg / dl).
First, at the beginning of this century, glucose sensors appeared which recorded tissue glucose over 72 hours, but did not display this to the user in real time. This enabled users and their professional advisors to retrospectively look at the course and draw conclusions for future therapy (Figure). There are several scientific studies in which a group of sensor users was compared to a similar comparison group after a random selection process ( randomized controlled study , RCT). The target values of such studies are usually the quality of the metabolic control ( HbA1c value) as a surrogate parameter for secondary complications and the number of severe hypoglycaemia as a risk to life and limb and as a cost factor. A large, independent study from the USA showed no advantages of these parameters in adolescents and children; an absolute improvement in HbA1c of 0.5 percentage points in adults. The assumption can be derived from the data that the negative result in young people can be traced back to a short gestation period (= time of use), which in turn allows speculations about a high level of use and the resulting reduction in quality of life. In motivated adults, the sensor can be integrated into everyday life and the HbA1c reduced. Further studies will show which subgroup benefits particularly and thus justifies the currently still high costs (see also Glucowatch Biographer above).
Avoidance of hypoglycaemia with real-time sensors
A mathematical or statistical prediction of whether hypoglycaemia will occur can be derived from the continuous measurement curve . In such a case, an alarm function can warn the user and prompt them to take in carbohydrates ("eat glucose") or, for example, switch off the insulin pump during sleep (basal rate interruption). In a controlled study with 26 people with type 1 diabetes in two hospitals in the USA who wore the Abbott Freestyle Navigator, 84% of threatened hypoglycaemia could be avoided by interrupting the basal insulin supply. Here the forecast horizon was around 35 minutes. Such a prediction tool is generally seen as a safety component in a future artificial pancreas. A higher level of security can be achieved by shortening the pre-warning time while accepting more false positive alarms.
In general, the longer the pre-warning time is selected, the more time there is for corrective measures. With diabetes mellitus, such warning times are usually beyond 20 minutes. Disadvantages of a long forecast horizon are the lower sensitivity (hypoglycaemia is correctly predicted) and the lower specificity (false positive alarms, hypoglycaemia is forecast even though it does not occur at all). The patient must therefore specify setting parameters on the sensor that reflect their preferences (confidence in the prediction).
Trials on humans with an artificial pancreas
Historically, the demand for a functioning, continuously measuring glucose sensor arose from the conception of a closed control loop for needs-based insulin infusion (artificial pancreas).
The figure on the right shows the structure of a closed control loop. An insulin pump is controlled by a controller which calculates the infusion rate for insulin depending on the deviation of the current blood sugar value from the target value.
In terms of control technology, time delays in the measurement signal (glucose sensor and signal filter) as well as in the controlled variable (insulin absorption and delay in action) are problematic, the longer they are and the lower the signal-to-noise ratio . Both an intravenous measurement and an intravascular infusion (iv-iv system) or at least into the abdominal cavity ( intraperitoneal ) would therefore be optimal .
Because of the associated problems, the decision was made in favor of the safe but slow subcutaneous-subcutaneous solution. Here, the gain, especially in the differential component of the control algorithm, must be reduced. This has led to the fact that the insulin determined by the regulator alone could not adequately control the postprandial (= after the meal) blood sugar, so that one is currently experimenting with semi-closed control loops, which 15 minutes before the respective meal as about half of the mealtime insulin Advance the bolus (single dose) and then let the controller “take care of the rest”. As a preliminary interim goal, the aim is to be able to set a person with such an artificial pancreas hypoglycemia- free at night and to be able to guide him out of the night with an acceptable blood glucose value.
Dissemination of commercial systems
Two national registers of children and adolescents under 18 years of age from Germany / Austria (DPV) and the USA (T1DX) show an increase in the use of rtCGM and FGM between 2011 and 2016 from 3% to approx. 16%. The mean HbA1c values as quality parameters of metabolic control changed only insignificantly in Europe during this period (7.9% → 7.8%) and even worsened slightly in the USA (8.5% → 8.8%). There is an indication in both registers that CGM / FGM users have a better mean HbA1c value (Europe 7.85% vs. 7.55%). Such register data are associations with no causal evidence for the effect.
Future developments
Ultimately, an artificial pancreas should record continuously measuring sensors. This is still at the experimental stage. Fully implantable sensors for subcutaneous measurement are about to be launched in Europe. Sensors that could measure glucose non-invasively with electromagnetic radiation, especially light, have so far only brought it to market shortly. Ultimately, there was a lack of accuracy and reproducibility of the results. Even when measuring glucose in other compartments of the body, e.g. B. intravenously in the operating room or in an intensive care unit, it has not yet resulted in a commercial system.
Practical aspects
- The approval for most CGM systems only allows parallel measurement; therapeutic decisions must then still be based on individual measurements with a conventional blood glucose meter. Safety studies with newer, higher-precision devices increasingly permit a formal suspension of parallel measurement for therapeutic purposes (see table)
- For almost all commercial sensor types, measurements with conventional glucose meters are also required for calibration purposes at least once a day. They are the greatest source of error for incorrect continuous measurement results from the sensor and should be carried out with great care. (See table)
- Correct use of sensors requires knowledge that can be acquired in training courses offered by diabetes clinics and specialist practices. In some cases, dose recommendation programs based on real-time sensor measurements (possibly also the trend arrows) also support.
- Due to the latency time between tissue and blood, the sensor inevitably measures a value that is too low when the blood sugar rises and too high when it falls. If the values are more or less stable, the difference is minimal and it makes sense to carry out a calibration.
Application in veterinary medicine
Initial studies on the continuous recording of the sugar level in animals are available in dogs with diabetes . The values of these systems deviate from the measurement data collected with classic blood sugar devices, but it is still possible to set the insulin dose on the basis of such continuous measurements. The extent to which temporary hypoglycaemia or a Somogyi effect can be reliably detected with this must be validated by further studies.
Individual evidence
- ↑ Updike, SJ and Hicks, GP: The enzyme electrode, a miniature chemical tranducer using immobilized enzyme activity. in Nature 214 pp. 986-988 (1967).
- ^ Clark LC Jr, Lyons C: Electrode systems for continuous monitoring in cardiovascular surgery . Ann NY Acad Sci 1962; 102: 29-45
- ↑ M. Shichiri et al .: Long-Term Glycemic Control with a portable Artificial Endocrine Pancreas in Pancreatomized Dogs. In: Artificial Systems for Insulin Delivery. Ed. By Brunetti et al. 1983: 445-455
- ↑ uni-ulm.de , accessed on May 25, 2017.
- ^ Pfeiffer, EF, The "Ulm Sugar Clock System" and its consequences . Horm Metab Res, 1994. 26 (11): p. 510-4
- ^ X. Wang et al .; Long-Term Home Study on Nocturnal Hypoglycemic Alarms Using a New Fully Implantable Continuous Glucose Monitoring System in Type 1 Diabetes; Diabetes Technology Therapeutics 17 (2015)
- ↑ On the way to a new diabetes care In: roche.de , accessed on May 25, 2017
- ↑ J. Lipson et al., Requirements for Calibration in Noninvasive Glucose Monitoring by Raman Spectroscopy; J Diabetes Sci Technol 2009; 3 (2): 233-241
- ↑ Pleitez et al., In Vivo Noninvasive Monitoring of Glucose Concentration in Human Epidermis by Mid-Infrared Pulsed Photoacoustic Spectroscopy; Anal. Chem., 2013, 85: 1013-1020
- ↑ D. Cook et al .: Randomized controlled trial to assess the impact of continuous glucose monitoring on HbA1c in insulin-treated diabetes (MITER Study) ; Diabetic Medicine, 26, 540-547
- ↑ DirecNet Study Group: A Randomized Multicenter Trial Comparing the GlucoWatch Biographer With Standard Glucose Monitoring in Children With Type 1 ; Diabetes Diabetes Care , Volume 28, Issue 5, May 2005
- ↑ Senseonics Reports Topline Accuracy Results from US Pivotal Study of Ever Sense CGM system. Accessed July 12, 2018 .
- ↑ Damiano ER. et al: A Comparative Effectiveness Analysis of Three Continuous Glucose Monitors: The Navigator, G4 Platinum, and Enlite; J. Diab Sci.Tech 2014
- ↑ Christiansen, M. et al. Accuracy of a Fourth Generation Subcutaneous Continuous Glucose Sensor; Diabetes Technol. Ther. 2017, p. 446
- ^ Agency for Quality and Accreditation in Health Care and Social Welfare (AAZ), Main Association of Austrian Social Security Institutions (HVB), The Norwegian Institute of Public Health (NIPHNO). Continuous glucose monitoring (CGM real-time) and flash glucose monitoring (FGM) as personal, standalone systems in patients with diabetes mellitus treated with insulin. Joint assessment. Zagreb: EUnetHTA; 2018. Report No .: OTJA08. [1] Retrieved January 18, 2019
- ↑ bfarm.de ( Memento of the original from May 21, 2017 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed on May 28, 2017.
- ↑ tuev-sued.de , accessed on May 28, 2017.
- ↑ IQWiG final report D12-01 Continuous interstitial glucose measurement (CGM) with real-time measuring devices for insulin-dependent diabetes mellitus ( memento of the original from July 6, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Federal Joint Committee: Resolution of the Federal Joint Committee on an amendment to the guidelines on methods of statutory health care: Continuous interstitial glucose measurement with real-time measuring devices (rtCGM) for therapy control in patients with insulin-dependent diabetes mellitus. (PDF; 481 kB) In: g-ba.de. June 16, 2016, accessed May 31, 2017 .
- ↑ Continuous interstitial glucose measurement. In: KVB Forum. No. 12, 2016, p. 192.
- ↑ DDG.de Statement by the German Diabetes Society (DDG) and its Working Group for Diabetological Technology (AGDT) on Flash Glucose Monitoring In: deutsche-diabetes-gesellschaft.de , accessed on May 25, 2017.
- ↑ BAG Archived copy ( Memento of the original dated November 27, 2017 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. accessed on November 24, 2017
- ↑ JDRF Study Group Continuous Glucose Monitoring in NEJM 2008
- ↑ U. Thurm, B. Gehr: CGM and insulin pump primer . 1st edition 2011, Kirchheim-Verlag, Mainz; ISBN 978-3-87409-509-9
- ↑ Buckingham, Bruce: Prevention of nocturnal hypoglycemia using predictive alarm algorithms and insulin pump suspension , Diabetes Care 2010 p. 2013.
- ↑ Cameron et al .; statistical hypo prediction; Journal of Diabetes Science and Technology Volume 2 (4), 2008
- ↑ SA Weinzimmer et al .: Fully Automated Closed-Loop Insulin Delivery Versus Semiautomated Hybrid Control in Pediatric Patients With Type 1 Diabetes Using an Artificial Pancreas , Diabetes Care 31: 934-939, 2008
- ↑ DeSalvo et al. Pediatric diabetes. 2018; 19: 1271–1275.DOI: 10.1111 / pedi.12711
- ↑ Gough DA, Kumosa LS, Routh TL, Lin JT, Lucisano JY. Function of an implanted tissue glucose sensor for more than 1 year in animals; Sci Transl Med.; 2:42 (2010)
- ↑ Ciudin A, Hernandez C, Simo R .; Non-invasive methods of glucose measurement: current status and future perspectives; Curr Diabetes Rev. 8: 48-54 (2012)
- ↑ Lisa Voigt: Flash glucose monitoring system - an alternative to capillary blood sugar measurement. In: Kleintierpraxis Volume 64, Issue 6, 2019, pp. 348–358.

