Ketorolac
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ketorolac | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 15 H 13 NO 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 255.27 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.33 g cm −3 |
||||||||||||||||||
| Melting point |
160.5 ° C |
||||||||||||||||||
| boiling point |
493.2 ° C |
||||||||||||||||||
| pK s value |
3.49 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ketorolac is a pyrrole derivative . It is used in medicine as a nonsteroidal anti-inflammatory drug ( NSAID ) and as a non-opioid analgesic . The active ingredient was patented by Syntex in 1978 .
application areas
The distribution of ketorolac for the treatment of postoperative pain ( Toratex ) was discontinued after the occurrence of fatal gastrointestinal bleeding in Germany in 1993, as well as in France. In Great Britain, the period of use has been limited to a few days and is generally not approved for children under one year of age.
In ophthalmology, ketorolac is used in the form of eye drops or a solution to be administered intraocularly to reduce and prevent inflammation and associated symptoms after eye surgery. Its salt with trometamol is used for this.
Type and duration of the effect
Ketorolac belongs to the class of non-opioid analgesics and is used in post-operative pain relief, post-traumatic pain relief, and severe low back pain. If there is severe postoperative pain, additional medication is required.
The effect occurs after oral administration after about 30 minutes and usually lasts for six hours.
The elimination half-life is between five to seven hours. This time can be extended by various environmental influences. On the one hand, the time in children is generally longer than in adults. On the other hand, kidney dysfunction leads to an extension of up to 19 hours. In addition, the ( S ) -enantiomer has a longer elimination half-life than the ( R ) -enantiomer.
Distribution in the body
Ketorolac can be given orally or intravenously for the management of post-operative pain. The substance is converted into glucuronic acid conjugates or p-hydroxyketorolac in the liver and then disposed of via the kidneys. 75% of the dose can be found in the urine within 7 hours.
Stereochemistry
Ketorolac contains a stereocenter and consists of two enantiomers . This is a racemate , i.e. a 1: 1 mixture of ( R ) and ( S ) form:
| Enantiomers of ketorolac | |
|---|---|
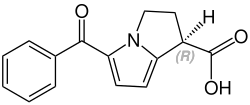 ( R ) -Ketorolac |
 ( S ) -Ketorolac |
Finished medicinal products
- KetoVision (D)
- In fixed combination with phenylephrine : Omidria (EU)
Individual evidence
- ↑ a b c d data sheet (R) -Ketorolac from Sigma-Aldrich , accessed on December 6, 2017 ( PDF ).
- ↑ A. Habibi Yangjeh, E. Pourbasheer, M. Danandeh-Jenagharad (ed.): Prediction of melting point for drug-like compounds using principal component-genetic algorithm-artificial neural network . In: Bulletin of the Korean Chemical Society , Vol. 29, No. 4, 2008, pp. 833-841.
- ↑ a b c Entry on Ketorolac. In: Römpp Online . Georg Thieme Verlag, accessed on July 12, 2019.
- ↑ There is not yet a harmonized classification for this substance . A labeling of 5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on December 6, 2017, is reproduced from a self-classification by the distributor .
- ↑ a b c d e f g R. B. Twycross, A Wilcock, S. Charlesworth, A. Dickman (Eds.): Palliative Care Formulary . Radcliff, 2006, ed. 4, ISBN 1-85775-511-1 , p. 154.
- ↑ a b c Rote Liste Service GmbH (Ed.): Rote Liste 2017 - drug directory for Germany (including EU approvals and certain medical devices) . Rote Liste Service GmbH, Frankfurt / Main, 2017, edition 57, ISBN 978-3-946057-10-9 , p. 193.
- ↑ Sale of Ketorolac (TORATEX) stopped . In: arznei-telegramm (at) 1993; No. 8, p. 84.
- ↑ a b c d e f B. Zernikow (Hrsg.): Pain therapy for children, adolescents and young adults . Springer Medizin Verlag, Heidelberg, 2009, edition 4, ISBN 978-3-540-74064-3 , pp. 108, 109.
