Kleptoplastid
Kleptochloroplasts or kleptoplastids are chloroplasts that are ingested by organisms and temporarily used photosynthetically ( mixotrophy ) or, if necessary, digested later when there is a lack of food. In contrast to the plastids of green algae and higher plants , which have achieved their plastids through endosymbiosis , they are not passed on to the offspring. The kleptoplastids usually last only a few days and are then replaced. The type of use and the stability of the ingested chloroplasts, however, vary greatly between the groups of organisms.
The selective preservation of kleptoplastids is used within the framework of the endosymbiont theory to explain the formation of chloroplasts from originally free-living cyanobacteria , cryptophytes or haptophytes .
Dinoflagellates
Examples of kleptoplasty can be found in the unicellular dinoflagellates . The heterotrophic species of the genus Dinophysis, for example, use the phycobilin- containing chloroplasts of their prey (cryptophytes). It is therefore not a common endosymbiosis, since apparently only the chloroplast of the cryptophytes is absorbed, without the nucleomorph and the two outer membranes, so that only a two-layer chloroplast remains. However, cryptophyte chloroplasts require that their nucleomorph be preserved in order to be able to survive and reproduce in the long term. Dinophysis species grown in isolation in cell culture cannot survive. It therefore seems possible (although not yet confirmed) that the dinophysis chloroplast is a kleptoplastid. This would mean that the Dinophysis chloroplasts wear out over time and the Dinophysis single cells constantly have to take in new cryptophytes in order to replace used chloroplasts with new ones. The Dinophysyis kleptoplasts can persist for up to two months. In other dinoflagellates such as Gymnodinium spp. and Pfiesteria piscicida , the cleptoplasts are photosynthetically active for only a few days. The kleptoplasts of mixotrophic ciliates of the genus Mesodinium ( M. rubrum ) can also become prey for Dnophysis ( D. acuminata ).
Ciliophora
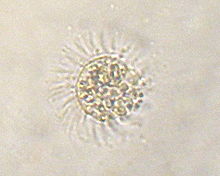
Myrionecta rubra is an eyelash animal ( Ciliophora ) that steals chloroplasts from the Cryptophyte Geminigera cryophila . M. rubra is itself a victim of kleptopastia when it falls prey to dinoflagellates of the genus Dinophysis .
Ciliates of the genus Strombidium rob chloroplasts of the alga Ulva . In the species S. tintinnodes (syn. S. oculatum ) kleptoplastidy of eye-spots has been found, see eye-spot §Strombidium .
The ciliate mesodinium robs red chloroplasts from cryptophytes .
Karyocleptia
Karyokleptia is a related process that also preserves the prey's cell nuclei. This was also described for the first time with M. rubra .
Foraminifera
Some chamberlings ( Foraminifera ) of the genera Bulimina , Elphidium , Haynesina , Nonion , Nonionella , Nonionellina , Reophax , Stainforthia and others rob chloroplasts from diatoms. In contrast, some foraminifera contain photosynthetically active dinoflagellates ( zooxanthellae ) as endosymbionts.
Sea snails


C = chloroplast,
N = cell nucleus (nucleus).
Electron micrograph: scale 3 µm.
The highest observed stability of the ingested chloroplasts is shown by the kleptochloroplasts of the bright green colored sea snail Elysia chlorotica . The animal ingests the alga Vaucheria litorea , digests most of the cell body and integrates the plastids into the epithelial cells of their digestive tract by phagocytosis . The organelle even continues to express plastid genes . In the aquarium the snail survives for eight to nine months without food, only through exposure to light, which corresponds to the typical lifespan in the wild. Some of the genes from the cell nuclei of the food were even transferred to the snails, which is why the chloroplasts can be supplied with proteins that are essential for them. Other snails of the families Conchoidea , Stiligeroidea and Elysioidea can also ingest chloroplasts in this way, but the stability of the kleptoplasts of E. chlorotica is not achieved anywhere else with about 10 months as far as is known.
The sea snails (Sacoglossa) of the Pacific can also ingest chloroplasts from algae and store them in their midgut gland or their skin. Some other marine snails eat corals , which in turn carry photosynthetic algae.
Unlike the gastrointestinal snails, the green hydra ( Hydrozoa ) lives in symbiosis with the ingested algae. The same applies to some Acoelomorpha z. B. the genus Waminoa , which live in symbiosis with zooxanthellae and, among other things, feed on their photosynthetic products ; Symsagittifera and also Convoluta (both Convolutidae ) live z. B. in symbiosis with the unicellular green alga Tetraselmis convolutae . This shows that from the outside - for example by the green color of a marine animal - it is not possible to tell whether there is a kleptoplasty or a symbiosis. Also, some types of sea slugs ( nudibranchs ) live in symbiosis with photosynthetic driving dinoflagellates (zooxanthellae so called) who are in their digestive diverticula are; so they are also 'solar powered'
The gastropod snails are thus the only known animals ( Metazoa ) in which kleptoplastidia is observed. However, the transition is fluid, as the example of Elysia shows.
See also
Individual evidence
- ^ Alf Skovgaard: Role of chloroplast retention in a marine dinoflagellate . In: Aquatic Microbial Ecology . 15, 1998, pp. 293-301. doi : 10.3354 / ame015293 .
- ↑ Richard G. Dorrell, Christopher J. Howe: Integration of plastids with their hosts: Lessons learned from dinoflagellates . In: Proceedings of the National Academy of Sciences . 112, No. 33, August 18, 2015, ISSN 0027-8424 , pp. 10247-10254. bibcode : 2015PNAS..11210247D . doi : 10.1073 / pnas.1421380112 . PMID 25995366 . PMC 4547248 (free full text).
- ↑ Charles F. Delwiche: Tracing the thread of plastid Diversity Through the Tapestry of Life . In: The American Naturalist , Vol. 154, Supplement: Evolutionary Relationships Among Eukaryotes, October 1999, pp. S164-S177, doi: 10.1086 / 303291 .
- ↑ Torstein Tengs, Ole J. Dahlberg, Kamran Shalchian-Tabrizi, Dag Klaveness, Knut Rudi, Charles F. Delwiche, Kjetill S. Jakobsen: Phylogenetic analyzes indicate that the 19'Hexanoyloxy-fucoxanthin-containing dinoflagellates have tertiary plastids of haptophyte origin . In: Molecular Biology and Evolution . 17, No. 5, 2000, pp. 718-29. doi : 10.1093 / oxfordjournals.molbev.a026350 . PMID 10779532 .
- ↑ Patrick J. Keeling: Diversity and evolutionary history of plastids and their hosts . In: American Journal of Botany . 91, No. 10, 2004, pp. 1481-93. doi : 10.3732 / ajb.91.10.1481 . PMID 21652304 .
- ↑ Jacques Joyard, Maryse A. Block, Roland Douce: Molecular aspects of plastid envelope biochemistry . In: Eur. J. Biochem. . 199, No. 3, 1991, pp. 489-509. doi : 10.1111 / j.1432-1033.1991.tb16148.x . PMID 1868841 .
- ↑ Kiyotaka Takishitaa, Kazuhiko Koikeb, Tadashi Maruyamaa, Takehiko Ogatab: Molecular Evidence for Plastid Robbery (Kleptoplastidy) in Dinophysis, a Dinoflagellate causing Diarrhetic Shellfish Poisoning . In: Protist , 2002, Vol. 153, pp. 293-302, PMID 12389818 .
- ↑ Jeremiah D. Hackett, Donald M. Anderson, Deana L. Erdner, Debashish Bhattacharya: Dinoflagellates: A remarkable evolutionary experiment . In: American Journal of Botany . 91, No. 10, 2004, pp. 1523-34. doi : 10.3732 / ajb.91.10.1523 . PMID 21652307 .
- ↑ JoAnn M. Burkholder: A 'poisonous alga' with many stealth caps , Spektrum.de from January 1, 2000
- ↑ Gast RJ, Moran DM, Dennett MR, Caron DA: Kleptoplasty in an Antarctic dinoflagellate: caught in evolutionary transition? . In: Environ. Microbiol. . 9, No. 1, January 2007, pp. 39-45. doi : 10.1111 / j.1462-2920.2006.01109.x . PMID 17227410 .
- ↑ Minnhagen S, Carvalho WF, Salomon PS, Janson S: Chloroplast DNA content in Dinophysis (Dinophyceae) from different cell cycle stages is consistent with kleptoplasty . In: Environ. Microbiol. . 10, No. 9, September 2008, pp. 2411-2417. doi : 10.1111 / j.1462-2920.2008.01666.x . PMID 18518896 .
- ↑ A. Mitra (2019), pp. 54f and 57
- ^ Matthew D. Johnson, David Oldach, F. Delwiche Charles, Diane K. Stoecker: Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra . In: Nature . 445, No. 7126, January 2007, pp. 426-428. doi : 10.1038 / nature05496 . PMID 17251979 .
- ↑ G. Nishitani, S. Nagai, K. Baba, S. Kiyokawa, Y. Kosaka, K. Miyamura, T. Nishikawa, K. Sakurada, A. Shinada, T. Kamiyama: High-level congruence of Myrionecta rubra prey and Dinophysis species plastid identities as revealed by genetic analyzes of isolates from Japanese coastal waters . In: Applied and Environmental Microbiology . 76, No. 9, 2010, pp. 2791-2798. doi : 10.1128 / AEM.02566-09 . PMID 20305031 . PMC 2863437 (free full text).
- ^ A. Mitra (2019), p. 57
- ↑ Strombidium oculatum Gruber, 1884 , on: World Register of Marine Species (WoRMS)
- ↑ David JS Montagnes, Chris D. Lowe, Alex Poulton, Per R. Jonsson: Redescription of Strombidium oculatum Gruber 1884 (Ciliophora, Oligotrichia) , in: The Journal of Eukaryotic Microbiology, July 12, 2015, doi: 10.1111 / j.1550 -7408.2002.tb00379.x
- ^ E. Fauré-Fremiet: The Origin of the Metazoa and the Stigma of the Phytoflagellates , in: Journal of Cell Science 1958, pp. 3-99: pp. 123-129; PDF1 @paperity
- ↑ A. Mitra (2019), pp. 54–56
- ↑ Matthew D. Johnson, David Oldach, et al. : Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra . In: Nature . 445, No. 7126, Jan 25, 2007, pp. 426-428. doi : 10.1038 / nature05496 . PMID 17251979 .
- ↑ Joan M. Bernhard, Samuel S. Bowser: Benthic foraminifera of dysoxic sediments: chloroplast sequestration and functional morphology . In: Earth Science Reviews . 46, No. 1-4, 1999, pp. 149-165. doi : 10.1016 / S0012-8252 (99) 00017-3 .
- ↑ Catherine Brahic: Solar-powered sea slug harnesses stolen plant genes . New Scientist . November 24, 2008. Retrieved April 6, 2019.
- ↑ CC Mujer, DL Andrews et al. : Chloroplast genes are expressed during the intracellular symbiotic association of Vaucheria litorea with the sea slug Elysia chlorotica . In: Proc Natl Acad Sci USA 93 (22), October 29, 1996, pp. 12333-12338, doi: 10.1073 / pnas.93.22.12333
- ↑ ME Rumpho, EJ Summer, JR Manhart: Solar-powered sea slugs. Mollusc / algal chloroplast symbiosis . In: Plant Physiology . 123, No. 1, May 2000, pp. 29-38. doi : 10.1104 / pp.123.1.29 . PMID 10806222 . PMC 1539252 (free full text).
- ↑ Muscatine L, Greene RW: Chloroplasts and algae as symbionts in molluscs (= International Review of Cytology), Volume 36 1973, ISBN 978-0-12-364336-0 , pp. 137-169, doi : 10.1016 / S0074-7696 (08) 60217-X . PMID 4587388
- ↑ Symbio: Introduction-Kleptoplasty . University of Maine. Archived from the original on December 2, 2008. Retrieved April 6, 2019.
- ↑ Rumpho ME, Worful JM, Lee J, Kannan K, Tyler MS, Bhattacharya D, Moustafa A, Manhart JR: Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica . In: Proceedings of the National Academy of Sciences of the United States of America . 105, No. 46, November 2008, pp. 17867-17871. bibcode : 2008PNAS..10517867R . doi : 10.1073 / pnas.0804968105 . PMID 19004808 . PMC 2584685 (free full text).
- ^ SK Pierce, SE Massey, JJ Hanten, and NE Curtis: Horizontal Transfer of Functional Nuclear Genes Between Multicellular Organisms . In: Biol. Bull. . 204, No. 3, June 1, 2003, pp. 237-240. doi : 10.2307 / 1543594 . PMID 12807700 . , JSTOR 1543594
- ^ W. Probst, European University of Flensburg, §Plant animals and kleptoplasts
- ↑ Händeler K., Grzymbowski YP, pitcher PJ & Wägele H. (2009) "functional chloroplasts in metazoan cells - a unique evolutionary strategy in animal life". Frontiers in Zoology 6 28 . doi: 10.1186 / 1742-9994-6-28 .
literature
- Aditee Mitra: Marine Biology - The Best of Two Worlds , Spectrum of Science, April 2019, pp. 54–60
- New predator category: Kleptoprädatoren , on: Wissenschaft.de from November 1, 2017; Trevor J. Willis et al. : Kleptopredation: a mechanism to facilitate planktivory in a benthic mollusc , in: Biology Letters from November 1, 2017, doi: 10.1098 / rsbl.2017.0447 . (This is related behavior, especially with Dinophysis )

