Carbonic acid ethyl methyl ester
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
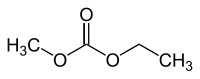
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Carbonic acid ethyl methyl ester | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 8 O 3 | ||||||||||||||||||
| Brief description |
colorless liquid with a sweet odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 104.10 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.01 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−14 ° C |
||||||||||||||||||
| boiling point |
107 ° C (1016 hPa) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.378 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Carbonic acid ethyl methyl ester is an organic chemical compound . It is a highly flammable, colorless liquid .
In a mixture with ethylene carbonate or propylene carbonate , the compound is used in non-aqueous electrolyte solutions for lithium batteries and lithium-ion accumulators .
Individual evidence
- ↑ a b c d Entry on ethyl methyl carbonate in the GESTIS substance database of the IFA , accessed on January 20, 2020(JavaScript required) .
- ↑ a b c data sheet ethyl methyl carbonate from Sigma-Aldrich , accessed on June 18, 2013 ( PDF ).
- ↑ a b W. M. Haynes, David R. Lide, Thomas J. Bruno: CRC Handbook of Chemistry and Physics 2012–2013 . CRC Press, 2012, ISBN 978-1-4398-8049-4 , pp. 3–262 ( limited preview in Google Book search).
- ↑ Michael S. Ding, Kang Xu, T. Richard Jow: Liquid-Solid Phase Diagrams of Binary Carbonates for Lithium Batteries . In: Journal of The Electrochemical Society . tape 147 , no. 5 , January 5, 2000, pp. 1688-1694 , doi : 10.1149 / 1.1393419 .
- ↑ Chris K. Dyer, Patrick T. Moseley, Zempachi Ogumi, David AJ Rand, Bruno Scrosati : Encyclopedia of Electrochemical Power Sources . Newnes, 2013, ISBN 0-444-52745-1 , pp. 76 ( limited preview in Google Book search).
- ↑ George E Blomgren: Electrolytes for advanced batteries . In: Journal of Power Sources . tape 81-82 , September 1999, pp. 112-118 , doi : 10.1016 / S0378-7753 (99) 00188-3 .
