Copper hexafluorosilicate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
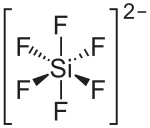
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Copper hexafluorosilicate | |||||||||||||||
| Molecular formula | Cu [SiF 6 ] | |||||||||||||||
| Brief description |
pale blue solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 205.62 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Copper hexafluorosilicate is an inorganic chemical compound of copper from the group of hexafluorosilicates .
Extraction and presentation
Copper hexafluorosilicate can be obtained by reacting hexafluorosilicic acid with copper (II) oxide .
properties
As a hexahydrate, copper hexafluorosilicate is a pale blue, crystalline powder that weathers in air and dissolves in moist air . It has a trigonal crystal structure with the space group R 3 (space group no. 148) and decomposes into sulfuric acid and sodium hydroxide . The tetrahydrate has a monoclinic crystal structure with the space group P 2 1 / c (space group no. 14) . The compound decomposes when heated.
use
Copper hexafluorosilicate was previously used as a wood preservative. It is used as a component of so-called CKF and CKFZ salts (active ingredients for wood preservatives which, in addition to chromium or copper salts, also contain fluorine compounds such as copper hexafluorosilicate or zinc hexafluorosilicate ). Copper hexafluorosilicate also serves as a hardener for white marble.
Individual evidence
- ↑ a b c d e f R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 440 ( limited preview in Google Book Search).
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 93rd Edition . CRC Press, 2016, ISBN 978-1-4398-8050-0 , pp. 62 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Martin Bertau, Armin Müller, Peter Fröhlich, Michael Katzberg: Industrielle Inorganische Chemie . John Wiley & Sons, 2013, ISBN 978-3-527-33019-5 , pp. 114 ( limited preview in Google Book search).
- ↑ MJR Clark, JE Fleming, H. Lynton: Crystal and molecular structure of CuSiF 6 • 4H 2 O. In: Canadian Journal of Chemistry. 47, 1969, p. 3859, doi : 10.1139 / v69-642 .
- ^ A b Michael D. Larrañaga, Richard J. Lewis, Sr., Robert A. Lewis: Hawley's Condensed Chemical Dictionary . John Wiley & Sons, 2016, ISBN 978-1-118-13515-0 , pp. 371 ( limited preview in Google Book search).
- ↑ Spektrum.de: Fluorosilicate - Lexicon of Chemistry - Spectrum of Science , accessed on November 24, 2016
- ^ Konrad Zilch, Claus Jürgen Diederichs, Rolf Katzenbach, Klaus J. Beckmann: Handbook for civil engineers, technology, organization and economy . Springer-Verlag, 2013, ISBN 978-3-642-14450-9 , pp. 198 ( limited preview in Google Book Search).
- ↑ schadstoffberatung.de: Holzschutz , accessed on November 23, 2016.
![{\ displaystyle \ mathrm {CuO + H_ {2} [SiF_ {6}] \ longrightarrow Cu [SiF_ {6}] + H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5d20b96b6b9a93127471b5ef34b7b0036dbfda72)