Lasmiditan
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Lasmiditan | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 19 H 18 F 3 N 3 O 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action |
Selective agonism at the 5-HT 1F receptor |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 377.36 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lasmiditan is a drug used to treat acute migraine attacks with or without aura . It is not suitable for migraine prevention. The substance from the Ditane group acts as an agonist on the serotonin receptor and can be used orally . In contrast to the triptans, which are also used in the treatment of migraines, Lasmiditan does not narrow the blood vessels.
Chemical properties
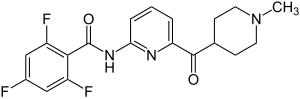
Lasmiditan is the first therapeutically used active ingredient from the Ditane group and, unlike the triptans also used in migraine treatment, has no tryptophan partial structure. Lasmiditan consists of three ring systems (trifluorobenzoic acid, pyridine-2-amine and N -methylpiperidine), which are connected to one another via functional groups.
The active ingredient is used pharmaceutically as lasmiditan hemisuccinate, i.e. the salt of succinic acid . Lasmitditan hemisuccinate is a white, crystalline powder, sparingly soluble in water, sparingly soluble in ethanol and soluble in methanol . Lasmiditan hydrochloride is described as another salt .
Pharmacological properties
Lasmiditan acts agonistically and selectively on the 5-HT 1F receptors . There is some evidence that its mechanism of action is based on reducing the release of neuropeptides , thereby inhibiting the conduction of pain, including those in the trigeminal nerve and ganglia. The selectivity of lasmiditan for the 5-HT 1F receptor is 440 times greater than that of the 5-HT 1B receptor, which is believed to be responsible for vasoconstrictive effects. The vasoconstricting effect on the peripheral vessels does not apply and Lasmiditan can also be used in patients with cardiovascular diseases.
Lasmiditan is orally effective; the most common undesirable effects observed were dizziness, fatigue, sensory disturbances and depression.
Admission
Lasmiditan was approved by the FDA in the United States for the acute treatment of migraines in October 2019 under the name Reyvow . The drug is a development of the Lilly subsidiary CoLucid Pharmaceuticals . An application for approval for the EU has been submitted, but approval is still pending.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ External identifiers of or database links to lasmiditan hemisuccinate : CAS number: 439239-92-6, EC number: 815-300-4, ECHA InfoCard: 100.249.353 , PubChem : 46927777 , ChemSpider : 30790735 , Wikidata : Q27292373 .
- ↑ Prescribing Information Reyvow Tablets 50 mg, 100 mg , Eli Lilly and Company, September 2019.
- ↑ External identifiers or database links to lasmiditan hydrochloride : CAS number: 613677-28-4, EC number: 947-092-7, ECHA InfoCard: 100.256.790 , PubChem : 17980336 , ChemSpider : 16472699 , Wikidata : Q90415167 .
- ↑ C. Müller: Lasmiditan for acute migraines - an alternative to triptans , Deutsche Apothekerzeitung, October 17, 2019.
- ↑ K. Gräfe: New option for migraine attacks , Pharmazeutische Zeitung, October 15, 2019.
- ↑ K. Gräfe: Ditane and Gepante against migraine attacks , Pharmazeutische Zeitung, October 14, 2019.
- ↑ FDA approves new treatment for patients with migraine , PM FDA of October 11, 2019, accessed November 6, 2019
- ↑ Lilly retrieves Lasmiditan from Ärzte Zeitung online, January 27, 2017.