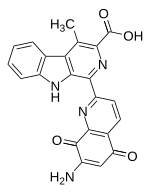
Lavendamycin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Lavendamycin | ||||||||||||
| Molecular formula | C 22 H 14 N 4 O 4 | ||||||||||||
| Brief description |
dark red crystals |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 398.37 g mol −1 | ||||||||||||
| Melting point |
> 300 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Lavendamycin is a naturally occurring chemical compound that can be isolated from the fermentation broth of the soil bacterium Streptomyces lavendulae . It has antibiotic properties and significant anti- proliferative effects against numerous cancer cell lines. However, the use of lavendamycin as a cytostatic in cancer therapy failed because of the poor solubility in water and the unspecific cytotoxicity of the compound.
discovery
Lavendamycin was first used in 1981 by Doyle et al. isolated from Streptomyces lavendulae . Since the compound did not crystallize , a direct elucidation of the molecular structure by means of a crystal structure analysis was not possible. Careful analysis using NMR , IR , UV / VIS spectroscopy and mass spectrometry enabled the compound to be assigned the pentacyclic structure, consisting of a β-carboline and a quinolinequinone unit.
Total syntheses
Its attractive biological properties and complex structure have made lavendamycin the target of numerous total syntheses . Just a few years after the structure was assigned by Doyle et al. The research groups of Kende, Hibino, Rao and Boger were able to independently develop total syntheses for this natural product . The discovery that analogs of lavendamycin are potent inhibitors of HIV reverse transcriptase led to numerous further attempts in the 1990s to develop an efficient access to lavendamycin. However, many reaction steps, low overall yields (0.5–2%) or poorly available starting materials make these syntheses unattractive for a systematic further development of lavendamycin. The total syntheses by Behforouz and Nissen, which allow a flexible construction of the lavendamycin skeleton in high yields, should therefore be emphasized.
Individual evidence
- ↑ a b entry on lavendamycin. In: Römpp Online . Georg Thieme Verlag, accessed on July 18, 2011.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ TW Doyle, DM Balitz, RE Grulich, DE Nettleton, SJ Gould, C. Tann, AE Moews, Tetrahedron Lett. , 1981 , 22 , 4595-4598.
- ↑ DM Balitz, JA Bush, WT Bradner, TW Doyle, FA O'Herron, DE Nettleton, J Antibiot , 1982 , 35 , 259-265.
- ↑ J.-F-Riou, P. Helissey, L. Grondard, S. Giorgi-Renault, Mol. Pharmacol. , 1991 , 40 , 699-706.
- ↑ N. Abe, Y. Nakakita, T. Nakamura, N. Enoki, H. Uchida, T. Takeo, M. Munekata, J. Antibiot. , 1993 , 46 , 1672-1677.
- ↑ G. Bringmann, Y. Reichert, VV Kane, Tetrahedron , 2004 , 60 , 3539-3574.
- ↑ AS Kende, FH Ebetino, Tetrahedron Lett. , 1984 , 25 , 923-926.
- ↑ S. Hibino, M. Okazaki, K. Sato, I. Morita, Heterocycles , 1983 , 20 , 1957-1958.
- ^ AVR Rao, SP Chavan, L. Sivadasan, Indian J. Chem. Sect. B , 1984 , 23B , 496-497.
- ↑ DL Boger, SR Duff, JS Panek, M. Yasuda, J. Org. Chem. , 1985 , 50 , 5790-5795.
- ^ MA Ciufolini, MJ Bishop, J. Chem. Soc., Chem. Commun. , 1993 , 1463-1464.
- ↑ Pedro Molina, Pilar M. Fresneda, Mercedes Cánovas, Tetrahedron Lett. , 1992 , 33 , 2891-2894; b) Pedro Molina, F. Murcia, Pilar M. Fresneda, Tetrahedron Lett. , 1994 , 35 , 1453-1456.
- ↑ P. Rocca, F. Marsais, A. Godard, G. Quéguiner, Tetrahedron Lett. , 1993 , 34 , 2937-2940.
- ↑ M. Behforouz, Z. Gu, W. Cai, MA Horn, M. Ahmadian, J. Org. Chem. , 1993 , 58 , 7089-7091.
- ↑ F. Nissen, H. Detert, Eur. J. Org. Chem. , 2011 , 2845-2853.