Manganese (III) acetylacetonate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
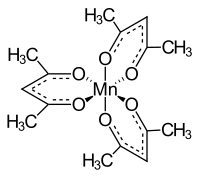
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Manganese (III) acetylacetonate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 15 H 21 MnO 6 | ||||||||||||||||||
| Brief description |
black solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 352.3 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
159–161 ° C (decomposition) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Manganese (III) acetylacetonate (Mn (acac) 3 ) is a complex of manganese in the +3 oxidation state, with acetylacetonate as a ligand .
Extraction and presentation
Manganese (III) acetylacetonate is made from a manganese (II) salt, potassium permanganate and acetylacetone.
The reaction initially produces manganese (II) acetylacetonate as an intermediate:
The reaction of Mn (II) and Mn (VII) produces Mn (III):
Overall reaction:
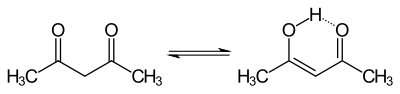
The acid formed is buffered by adding sodium acetate . Furthermore, it shifts the equilibrium in the enol-keto-tautomerism of acetyl acetone.
Due to the weakly basic property of the sodium acetate, the enol form is deprotonated and the acetylacetone is converted into its anionic form. It thus stabilizes the d 4 -configured manganese ion through complex formation.
properties
Manganese (III) acetylacetonate is a black, crystalline powder that is insoluble in water. According to the electronic structure, it forms a high spin complex . The structure is octahedrally distorted due to the Jahn-Teller effect . This can either be octahedral-compressed or octahedral-stretched. When stretched, the lengths of two Mn-O bonds are 2.12 Å, with the other four being only 1.93 Å. When compressed, the lengths of two Mn-O bonds are 1.95 Å while the other four bond lengths are 2.00 Å.
use
Manganese (III) acetylacetonate is used to oxidize phenols , β-dicarbonyl compounds and thiols .
literature
- J. Derek Woollins: Inorganic Experiments. Wiley-VCH, Weinheim 1994, ISBN 3-527-29253-5 , pp. 118-119.
- F. Albert Cotton, Geoffrey Wilkinson, Carlos A. Murillo, Manfred Bochmann: Advanced Inorganic Chemistry. 6th ed. Wiley-Interscience, New York 1999, ISBN 0-471-19957-5 .
Individual evidence
- ↑ a b c d e f Datasheet Manganese (III) acetylacetonate from Sigma-Aldrich , accessed on June 18, 2012 ( PDF ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1616-1617.