Mifamurtide
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
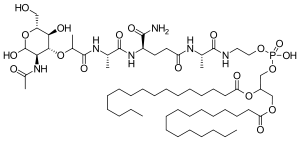
|
||||||||||
| General | ||||||||||
| Non-proprietary name | Mifamurtide | |||||||||
| Molecular formula | C 59 H 109 N 6 O 19 P (mifamurtide) | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| ATC code | ||||||||||
| Drug class | ||||||||||
| properties | ||||||||||
| Molar mass | 1237.50 g · mol -1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Mifamurtide (trade name Mepact ® ; manufacturer Takeda Pharmaceutical Company ) is a drug that is said to be used to treat osteosarcoma , a rare malignant bone tumor disease , in children, adolescents and young adults. Mifamurtide was classified as an orphan drug and approved for therapy by the European Commission in 2009 .
history
Mifamurtide was developed by Ciba-Geigy in the early 1980s for use in tumor therapy. After the first clinical studies , the rights to Jenner Biotherapies were sold in the 1990s . After mifamurtide was granted orphan drug status by the US Food and Drug Administration (FDA) in 2001 , IDM Pharma acquired the rights to mifamurtide and developed it further. In 2004 the European Medicines Agency also granted mifamurtide orphan drug status. After a failed application for approval in the USA in 2007, the European Commission granted IDM Pharma SAS a marketing authorization for mifamurtide (trade name: Mepact) in the European Union in 2009. In the same year IDM Pharma and Mepact were taken over by the pharmaceutical company Takeda.
Clinical information
Application areas (indications)
Mifamurtide is approved for the treatment of osteosarcomas in children, adolescents and young adults. There is insufficient experience for patients over 30 years of age.
Clinical studies
Mifamurtide was examined, among other things, in a phase III study in which 678 patients between the ages of 1 and 31 with highly malignant, non-metastatic osteosarcoma took part. After the tumor had been surgically removed, all patients received adjuvant chemotherapy in various combinations. Half of the patients were also treated with the immunomodulator mifamurtide: 68% of the patients (231 of 338) survived without the disease recurring under therapy with mifamurtide. The corresponding number in patients who did not receive mifamurtide was 61% (207 of 340). Compared to therapy without mifamurtide, treatment with mifamurtide reduced the risk of death by 28%.
Contraindications (contraindications)
Apart from a known hypersensitivity to the active substance, simultaneous treatment with are cyclosporine and other calcineurin inhibitors and treatment with high doses of NSAIDs as a contraindication .
Interactions
High-dose non-steroidal anti-inflammatory drugs inhibit the effects of mifamurtide on macrophages . Long-term use of immunosuppressive glucocorticoids also inhibits the stimulating effect of mifamurtide on the immune system. Ciclosporin and other calcineurin inhibitors can at least theoretically influence the function of splenic macrophages and mononuclear phagocytes. Lipophilic drugs such as doxorubicin should be administered separately in time. In contrast, mifamurtide shows no interactions with the cytochrome P450 enzyme system. Likewise, no interactions with cisplatin , ifosfamide and high-dose methotrexate could be detected.
Adverse drug effects
Mifamurtide shows characteristic side effects of drugs in active immunotherapy . Almost all patients (approx. 90%) suffered from fever and chills . The most common side effects, affecting around 50% of patients, include nausea, vomiting , fatigue, tachycardia, and headache . Very often (> 10%) dyspnoea , cough , sweating , hypertension , hypotension , dizziness , diarrhea, constipation, loss of appetite, abdominal pain, back pain, joint pain and muscle pain also occur.
pharmacology
Pharmacodynamics
The anti-tumor effect of mifamurtide is based on the activation of monocytes and macrophages . As a fully synthetic analog of muramyl dipeptide , the smallest naturally occurring immune-stimulating component of the cell wall of mycobacteria , Mifamurtide has similar immunostimulatory properties and simulating a bacterial infection. Its immune-stimulating effect is based on the interaction with NOD2 , a receptor that is mainly found on monocytes, dendritic cells and macrophages. This interaction leads to an increased formation and release of cytokines and adhesion molecules such as TNF-α , interleukin-1 , interleukin-6 , interleukin-8 , interleukin-12 , lymphocyte function-associated antigen-1 and ICAM-1 and to an activation of white blood cells. These are able to attack tumor cells , at least in vitro . The exact anti-tumor mechanism in vivo is not yet known.
Pharmacokinetics
After infusion of the mifamurtide administered in liposomal form, the drug is quickly removed from the plasma within a few minutes and accumulates in the liver , spleen , nasopharynx and thyroid gland . Its terminal half-life is about 18 hours. Cumulative effects after a second treatment after 11 to 12 weeks could not be observed.
chemistry
Chemical properties
Mifamurtide is a very lipophilic drug that is hardly soluble in water. As a phospholipid, it accumulates in the lipid bilayer of liposomes used as a vehicle.
synthesis
Two methods have been described for the production of mifamurtide. The first is based on the esterification of N -acetylmuramyl- L -alanyl- D -isoglutaminyl- L -alanine with N -hydroxysuccinimide and a subsequent condensation reaction with 2-aminoethyl-2,3-dipalmitoylglyceryl phosphoric acid. The second synthesis variant starts from N -acetylmuramyl- L -alanyl- D -isoglutamine and a reaction with L- alanyl-2-aminoethyl-2,3-dipalmitoylglycerylphosphoric acid after activation with hydroxysuccinylimine.
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Mifamurtide
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Frampton JE. Mifamurtide: a review of its use in the treatment of osteosarcoma. In: Pediatr Drugs 2010; 12: 141-153, PMID 20481644 .
- ↑ Ando K et al. Mifamurtide for the treatment of nonmetastatic osteosarcoma. In: Expert Opin Pharmacother . 2011; 12: 285-292, PMID 21226638 .
- ↑ a b c d e f g h i Summary of the European public assessment report (EPAR) for Mepact (mifamurtide). online (pdf; 132 kB).
- ↑ Mifamurtide: CGP 19835, CGP 19835A, L-MTP-PE, liposomal MTP-PE, MLV 19835A, MTP-PE, muramyltripeptide phosphatidylethanolamine . In: Drugs RD . 9, No. 2, 2008, pp. 131-5. PMID 18298131 .
- ↑ Fidler IJ et al. : Efficacy of liposomes containing a lipophilic muramyl dipeptide derivative for activating the tumoricidal properties of alveolar macrophages in vivo . In: J. Biol. Response Modif. . 1, 1982, pp. 43-55.
- ↑ Prous JR, Castaner J: ENV 2-3 / MTP-PE . In: Drugs Fut. . 14, No. 3, 1989, p. 220.
- ↑ Brundish DE, Wade R: Synthesis of N- [2-3H] acetyl-D-muramyl-L-alanyl-D-iso-glutaminyl-L-alanyl-2- (1 ', 2'-dipalmitoyl-sn-glycero -3'-phosphoryl) ethyl amide of high specific radioactivity. . In: J Label Compd Radiopharm . 22, No. 1, 1985, pp. 29-35.
