Monobrom German
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
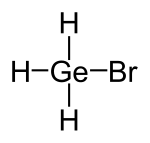
|
||||||||||
| General | ||||||||||
| Surname | Monobrom German | |||||||||
| Molecular formula | GeH 3 Br | |||||||||
| Brief description |
colorless liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 155.5 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
2.34 g cm −3 |
|||||||||
| Melting point |
−32 ° C |
|||||||||
| boiling point |
52 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Monobromgerman is a chemical compound from the group of Germans .
Extraction and presentation
Monobromgerman can be obtained by reacting Monogerman with bromine or silver bromide .
It is also possible to display it by reacting monogerman with boron tribromide .
properties
Monobromgerman is a colorless hydrolysis-sensitive liquid that slowly disproportionates at room temperature and can be stored at −78 ° C.
See also
Individual evidence
- ↑ a b c d e f g Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 729.
- ^ Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 470 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.


