NMR shift reagents
NMR shift reagents are chemical compounds that are used in NMR spectroscopy to either increase the resolution of the spectrum or, in the case of chiral shift reagents, to distinguish between enantiomers . There are three types of shift reagents: derivatization reagents, solvation reagents and lanthanoid shift reagents.
Derivatization reagents
Derivatization reagents are reacted with the analyte to form covalent bonds . Functional groups such as alcohols , amines or carboxylic acids in the analyte are a prerequisite for this . The derivatization creates a new molecule whose resonances in the NMR spectrum are different from the original substance. The resonances are shifted and thus better resolved.
Chiral derivatization reagents can be used to elucidate the structure and determine the optical purity of chiral compounds. The derivatization converts the enantiomers into diastereomers . Since diastereomers usually have different properties, they can be differentiated by NMR spectroscopy.
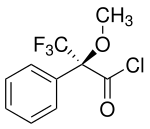
One of the best known chiral derivatizing reagents is Mosher acid . This can be reacted in the form of the acid chloride with amines and alcohols and thereby forms diastereomers. These can be examined both with 1 H-NMR spectroscopy and with the more sensitive 19 F spectroscopy .
Chiral Solvation Reagents
In contrast to the derivatization reagents, solvation reagents are not reacted with the analyte but, depending on the type of solvation reagent, only form interactions such as van der Waals forces , dipolar or ionic interactions and thus form a solvate . Racemic analytes, which are brought into an enantiomerically pure environment, behave like diastereomers, which can show different shifts in NMR. The only structural requirement for a chiral solvation reagent is enantiomeric purity. Solvation reagents can be divided into different groups:
- Low molecular weight solvation reagents such as Pirkles alcohol
- Solvation reagents that utilize an ion-pair effect
- molecular tweezers
- synthetic macrocycles
- Cyclodextrins
- Natural substances
The name suggests that the analytes are dissolved in the solvation reagent, but in practice a 2: 1 ratio of solvation reagent to analyte is usually used. The substance mixture is then dissolved in a deuterated solvent and examined.
Lanthanoid Shift Reagents
Lanthanoid shift reagents generally do not form a covalent bond to the substrate, so they do not have to be converted in a reaction, but can be mixed with the analyte in the NMR tube. The interaction with the analyte takes place via Lewis acid-base adducts . For this purpose, Lewis basic centers, such as e.g. B. alcohols, ethers, amines or thiols may be present. The shift of the resonances can be explained by the paramagnetism of the shift reagents. Since the shift intensity depends on the spatial structure of the analyte, structure elucidation can be carried out in this way. Common lanthanoid shift reagents are z. B. Eu (fod) 3 and Eu (dmp) 3 .
There are also chiral shift reagents, such as. B. Eu (tfc) 3 or Eu (hfc) 3 , which can be used to determine the absolute configuration.
Individual evidence
- ↑ a b c Sachin Rama Chaudhari, N Suryaprakash: Recent NMR methodological developments for chiral analysis in isotropic solutions . In: Journal of the Indian Institute of Science . tape 94 , no. 4 , October 29, 2014, p. 485-516 .
- ↑ James A. Dale, Harry S. Mosher: Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and .alpha.-methoxy-.alpha.-trifluoromethylphenylacetate (MTPA) esters . In: Journal of the American Chemical Society . tape 95 , no. January 2 , 1973, p. 512-519 , doi : 10.1021 / ja00783a034 .
- ↑ Lu Yang, Thomas Wenzel, R. Thomas Williamson, Melodie Christensen, Wes Schafer: Expedited Selection of NMR Chiral Solvating Agents for Determination of Enantiopurity . In: ACS Central Science . tape 2 , no. 5 , May 25, 2016, p. 332-340 , doi : 10.1021 / acscentsci.6b00062 , PMID 27280168 , PMC 4882744 (free full text).
- ↑ Federica Balzano, Gloria Uccello-Barretta, Federica Aiello: Chiral Analysis by NMR Spectroscopy: Chiral Solvating Agents . In: Chiral Analysis . Elsevier, 2018, ISBN 978-0-444-64027-7 , pp. 367-427 , doi : 10.1016 / b978-0-444-64027-7.00009-4 .
- ↑ William H. Pirkle, Dennis J. Hoover: NMR Chiral Solvating Agents . In: Topics in Stereochemistry . John Wiley & Sons, Inc., Hoboken, NJ, USA 2007, ISBN 978-0-470-14722-1 , pp. 263-331 , doi : 10.1002 / 9780470147221.ch4 .
- ^ Carlos FGC Geraldes: Lanthanides: Shift Reagents . In: Encyclopedia of Inorganic and Bioinorganic Chemistry . John Wiley & Sons, Ltd, Chichester, UK 2012, ISBN 978-1-119-95143-8 , pp. eibc2050 , doi : 10.1002 / 9781119951438.eibc2050 .