Neurin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
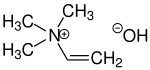
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Neurin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 13 NO | |||||||||||||||
| Brief description |
highly viscous liquid with a fish-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 103.16 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| Melting point |
<25 ° C |
|||||||||||||||
| solubility |
soluble in water and ethanol |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Neurin , also known as vinyl trimethylammonium hydroxide or trimethylvinylammonium hydroxide , is a poisonous alkaloid . It is formed as part of the corpse poison in putrefaction of protein , for example, in rotting muscle meat, the elimination of water from the choline . Neurin and choline are both quaternary ammonium compounds . A differentiation from neurin is the non-toxic neuridin (or spermine ).
Emergence
In putrefaction processes, the choline contained in almost all living things is transformed into neurin when heated and dehydrated (the anion was omitted in each case):
toxicology
In older studies from 1926 and 1935 on guinea pigs, mice and rabbits, neurin was highly toxic; the LD Lo values determined were between 30 and 100 mg / kg body weight of the animals used.
Individual evidence
- ↑ a b entry on Neurin. In: Römpp Online . Georg Thieme Verlag, accessed on September 30, 2014.
- ^ Entry on Neurin in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on November 27, 2018.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c d Abdernalden's Handbook of Biological Working Methods. Vol. 4, pp. 1289, 1935 .
- ^ A b Journal of Pharmacology and Experimental Therapeutics . Vol. 28, p. 367, 1926 .
