Niobium (IV) fluoride
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| __ Nb 4+ __ F - | |||||||
| Crystal system |
tetragonal |
||||||
| Space group |
I 4 / mmm (No. 139) |
||||||
| Lattice parameters |
a = 4.0876 (5) Å, c = 8.1351 (19) Å |
||||||
| Coordination numbers |
[6] Nb |
||||||
| General | |||||||
| Surname | Niobium (IV) fluoride | ||||||
| other names |
Niobium tetrafluoride |
||||||
| Ratio formula | NbF 4 | ||||||
| Brief description |
black solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 168.91 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| density |
4.01 g cm −3 |
||||||
| Melting point |
> 350 ° C (decomposition) |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Niobium (IV) fluoride is a chemical compound of niobium from the group of fluorides .
Extraction and presentation
Niobium (IV) fluoride can be obtained by decomposing Nb 2 F 5 at around 1000 ° C. or by reacting niobium (V) fluoride with niobium.
properties
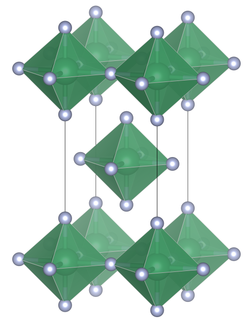
Niobium (IV) fluoride is a hygroscopic paramagnetic black solid. It has a tetragonal crystal structure of tin (IV) fluoride with the space group I 4 / mmm (space group no. 139) with the lattice constants a = 4.0876 (5) Å, c = 8.1351 (19) Å. The compound is stable in a vacuum up to about 300 ° C, but decomposes above 350 ° C into niobium (V) fluoride and niobium (III) fluoride .
Individual evidence
- ↑ a b c d William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, 2016, ISBN 978-1-4987-5429-3 , pp. 105 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Jascha Bandemehr, Matthias Conrad, Florian Kraus: Redetermination of the crystal structure of NbF 4 . In: Acta Crystallographica Section E Crystallographic Communications. 72, 2016, p. 1211, doi : 10.1107 / S2056989016012081 .
- ↑ Holleman / Wiberg: Subgroup elements, lanthanoids, actinides, transactinides Volume 2: Subgroup elements, lanthanoids, actinides, transactinides, appendices . Walter de Gruyter GmbH & Co KG, 2016, ISBN 978-3-11-049590-4 , p. 1836 ( limited preview in Google Book search).
- ^ Viktor Gutmann: Halogen Chemistry . Elsevier, 2012, ISBN 0-323-14847-6 , pp. 132 ( limited preview in Google Book search).
