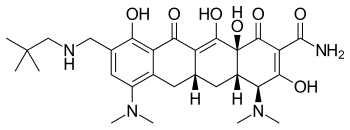
Omadacycline
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Omadacycline | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 29 H 40 N 4 O 7 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | 556.66 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Omadacycline (formerly PTK-0796) is a broad-spectrum antibiotic from the group of tetracyclines , which can be administered intravenously or in tablet form. Chemically, it is an aminomethylcycline.In the USA, it was approved by the FDA on October 3, 2018 under the name Nuzyra for the treatment of community-acquired pneumonia (CAP) and for acute skin and connective tissue infections .
discovery
At the "Tufts University School of Medicine" in Boston, USA , a team led by Mark L. Nelson has synthesized over 3000 new derivatives of tetracycline. Two compounds, omadacycline and sarecycline , were further investigated. Mohamed Ismail was also part of the team. They were supported by Kwasi Ohemeng and Laura Honeyman from Paratek Pharmaceuticals, Boston.
In Vitro Studies
In-vitro studies have shown that omadacycline is effective against a wide range of gram-positive bacteria and a certain number of gram-negative pathogens. The effectiveness is particularly important for the following problem germs:
- MRSA ( methicillin- resistant Staphylococcus aureus )
- Penicillin and multi-resistant Streptococcus pneumoniae
- VRE ( Vancomycin Resistant Enterococcus )
In vitro antibiotic activity of omadacycline has also been found in various gram negative aerobes and some anaerobes . The substance is also effective against atypical bacteria such as legionella and chlamydia . The in-vivo efficacy of omadacycline has been successfully confirmed by studies on infected mice. Omadacycline is metabolically stable. There is no significant metabolism. No interactions with metabolic enzymes or with cellular transporters could be determined either.
Mechanism of action
Similar to other tetracyclines, omadacycline inhibits bacterial protein synthesis. Omadacycline also showed an effect on two main types of tetracycline resistance: Eflux and ribosomal protection. With the Eflux, the bacteria get rid of the antibiotic by being transported to the outside. With ribosomal protection, a special mechanism prevents the tetracycline from attacking the bacterial, protein-synthesizing ribosomes.
Clinical results
A Phase II study evaluated the safety and effectiveness of omadacycline compared to linezolid in the treatment of complicated skin and connective tissue infections. Complicated skin and connective tissue infections are diseases that affect deep connective tissue layers of the skin or require surgical intervention, such as infected ulcers , burns or larger abscesses . In the study, which involved 11 centers in the United States, either omadacycline 100 mg intravenously daily or linezolid 600 mg intravenously twice daily. It was possible to switch the dose to 200 mg orally omadacycline or to 600 mg linezolid orally twice daily. Treatment with omadacycline was well tolerated and was found to be effective in treating complicated skin and connective tissue infections.
In June 2013, the US Food and Drug Administration ( FDA ) recognized omadacycline as a "qualified infectious disease product" in the treatment of acute skin and connective tissue infections as well as community-acquired pneumonia.
In June 2015, the OASIS-1 trial, a phase III non-inferiority study, began in 650 patients comparing omadacycline with linezolid in the treatment of acute skin and connective tissue infections. Of the study participants, 206 had inflammatory wound complications, 242 erysipelas or cellulitis, and 180 major abscesses . Proven pathogens were Staphylococcus aureus in 156 cases, of which 79 MRSA , Streptococcus anginosus in 47 cases, Streptococcus pyogenes 11 times and Enterococcus faecalis in 10 cases. The study confirmed that omadacycline is not inferior to linezolid (10% limit) in the treatment of acute skin and connective tissue infections. Infections with MRSA also showed clinical improvement in 83% after omadacycline treatment. The treatment was generally well tolerated. The most common side effects were gastrointestinal symptoms (e.g. nausea, vomiting, or diarrhea), which were slightly more common with omadacycline (18%) than with linezolid (15.8%).
A phase II study was started to test the value of omadacycline in the treatment of urinary tract infections . The results are expected at the end of 2019.
In the OASIS-2 study, the oral administration of omadacycline was tested in acute skin and connective tissue infections. This Phase III study compared omadacycline once daily with linezolid twice daily. In a joint evaluation of OASIS-1 and OASIS-2, the good efficacy of omadacycline and the non-inferiority to linezolid for both oral and intravenous treatment could be confirmed.
The effect of omadacycline in comparison to moxifloxacin in community-acquired pneumonia was examined in the OPTIC trial. In a randomized, double-blind, non-inferiority phase III study, 386 patients received omadacycline 100 mg intravenously twice on day 1, then once daily. Another 388 patients received 400 mg moxifloxazine intravenously daily. After three days of therapy, a switch from injection to tablets was allowed. The severity of the pneumonia was determined using the PSI score. 57% of the patients had pneumonia PSI Risk Class III, 28% Class IV. In addition to gram-positive bacteria such as Streptococcus pneumoniae and Staphylococcus aureus , gram- negative bacteria such as Haemophilus influencae and Klebsiella pneumoniae as well as atytic bacteria such as Mycoplasma pneumoniae , Legionella pneumoniae and Chlamydia pneumoniae were detected. The evaluation showed that omadacycline was not statistically inferior to moxifloxacin. However, 8 patients (2.1%) died in the omadacycline group, but only 4 (1%) in the moxifloxacin group. Therapy-related side effects, especially nausea and diarrhea, were more common after moxifloxacin than after omadacycline.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Laura Honeyman, Mohamed Ismail, Mark L Nelson, Beena Bhatia, Todd E. Bowser: Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline . In: Antimicrobial Agents and Chemotherapy . tape 59 , no. 11 , 2015, p. 7044-7053 , doi : 10.1128 / AAC.01536-15 , PMID 26349824 , PMC 4604364 (free full text).
- ^ Antibacterial Drug Development Task Force: Omadacycline Injection and Oral Products. US Food & Drug Administration, October 5, 2018, accessed March 5, 2019 .
- ↑ Ref: Mark L. Nelson and Kwasi Ohemeng: 4-dedimethylamino tetracycline compounds, United States (US) patent number 7,056,902 (2006)
- ^ S. Ken Tanaka, Stephen Villano: In Vitro and In Vivo Assessments of Cardiovascular Effects with Omadacycline . In: Antimicrobial Agents and Chemotherapy . tape 60 , no. 9 , 22 August 2016, p. 5247-5253 , doi : 10.1128 / AAC.00320-16 , PMID 27324778 , PMC 4997885 (free full text).
- ↑ Stephen Villano, Judith Steenbergen, Evan Loh: Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections . In: Future Microbiology . tape 11 , 2016, p. 1421-1434 , doi : 10.2217 / fmb-2016-0100 , PMID 27539442 .
- ↑ A B. Macone, BK Caruso, RG Leahy, J Donatelli, S Weir: In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline . In: Antimicrobial Agents and Chemotherapy . tape 58 , no. 2 , 2014, p. 1127–1135 , doi : 10.1128 / AAC.01242-13 , PMID 24295985 , PMC 3910882 (free full text).
- ↑ Jimmy Flarakos, Yancy Du, Helen Gu, Lai Wang, Heidi J Einolf: Clinical disposition, metabolism and in vitro drug-drug interaction properties of omadacycline . In: Xenobiotica; the Fate of Foreign Compounds in Biological Systems . tape 47 , no. 8 , 2017, p. 682-696 , doi : 10.1080 / 00498254.2016.1213465 , PMID 27499331 .
- ↑ Michael P Draper, S Weir, A Macone, J Donatelli, CA Trieber: Mechanism of Action of the Novel Aminomethylcycline Antibiotic Omadacycline . In: Antimicrobial Agents and Chemotherapy . tape 58 , no. 3 , 2014, p. 1279–1283 , doi : 10.1128 / AAC.01066-13 , PMID 24041885 , PMC 3957880 (free full text).
- ↑ Gary J Noel, Michael P Draper, Howard Hait, S Ken Tanaka, Robert D work: A Randomized, Evaluator-Blind, Phase 2 Study Comparing the Safety and Efficacy of Omadacycline to Those of Linezolid for Treatment of Complicated Skin and Skin Structure Infections . In: Antimicrobial Agents and Chemotherapy . tape 56 , no. 11 , 2012, p. 5650-5654 , doi : 10.1128 / AAC.00948-12 , PMID 22908151 , PMC 3486554 (free full text).
- ↑ Kathryn M Boxmeyer: Paratek Pharmaceuticals Announces FDA Grant of Qualified Infectious Disease Product (QIDP) Designation for Its Lead Product Candidate, Omadacycline - QIDP Status Designated for Both Intravenous and Oral Formulations -. SOURCE Paratek Pharmaceuticals, Inc., March 3, 2013, accessed March 5, 2019 .
- ↑ Don Seiffert: Paratek presents new trial data for antibiotic as late-stage trials continue. Boston Business Journal, November 4, 2016, accessed March 5, 2019 .
- ^ William O'Riordan, Sinikka Green, J Scott Overcash, Ivan Puljiz, Symeon Metallidis: Omadacycline for Acute Bacterial Skin and Skin-Structure Infections . In: New England Journal of Medicine . tape 380 , no. 6 , February 7, 2019, p. 528-538 , doi : 10.1056 / NEJMoa1800170 .
- ↑ Ben Strain: Paratek Pharmaceuticals Doses First Patient in Phase 2 Clinical Trial of Omadacycline in Acute Pyelonephritis, a Common Subset of Complicated Urinary Tract Infections. P&T Community, November 27, 2018, accessed March 5, 2019 .
- ↑ Michael Lampe, Hans Vitzthum: Paratek Initiates Phase 3 Study of Oral-only Omadacycline in ABSSSI. Paratek Pharmaceuticals, August 15, 2016, accessed March 5, 2019 .
- ↑ Fredrick M Abrahamian, George Sakoulas, Evan Tzanis, Amy Manley, Judith N Steenbergen: 1347. Omadacycline for Acute Bacterial Skin and Skin Structure Infections: Integrated Analysis of Randomized Clinical Trials . In: Open Forum Infectious Diseases . tape 5 , Suppl 1, November 26, 2018, p. S412 , doi : 10.1093 / ofid / ofy210.1178 , PMC 6253360 (free full text).
- ^ A b Roman Stets, Monica Popescu, Joven R Gonong, Ismail Mitha, William Nseir: Omadacycline for Community-Acquired Bacterial Pneumonia . In: New England Journal of Medicine . tape 380 , no. 6 , February 7, 2019, p. 517-527 , doi : 10.1056 / NEJMoa1800201 .
- ↑ MJ Fine, TE Auble, DM yealy, BH Hanusa, LA Weissenfeld: A prediction rule to identify low-risk patients with community-acquired pneumonia . In: The New England Journal of Medicine . tape 336 , no. 4 , January 23, 1997, p. 243-250 , doi : 10.1056 / NEJM199701233360402 , PMID 8995086 .