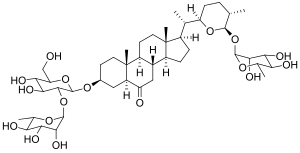
Osladin
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Osladin | |||||||||
| Molecular formula | C 45 H 74 O 17 | |||||||||
| Brief description |
colorless crystals |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 887.06 g · mol -1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
202-204 ° C |
|||||||||
| solubility |
very low in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Osladin (from the Czech osladič "spotted fern", to osladit "sweet") is a steroid saponin , which occurs in the rhizome of the common spotted fern ( Polypodium vulgare ).
The glycoside has a sweet taste, its sweetness is about 500 times that of sucrose . Hence the name angelic root for the rhizome of the fern. For toxicological reasons, however, osladin is not suitable as a sweetener .
Structure and sweetness
The substance was first isolated in 1971 and its structure as steroid saponin was clarified in 1975. After the first total synthesis in 1993 by Yamada and Nishizawa, however, the product was not found to be sweet-tasting. This led to the finding that the sweetness of the compound depends on the stereochemistry at carbon atoms 22, 25 and 26 ( tetrahydropyran ring with rhamnosyl residue at C-26), which had been neglected in the structure elucidation in 1975. The carbon atom at position 22 is in the R -, the C-25 in the S - and the C-26 in the R - configuration .
In addition to its toxicity, the use of osladin as a sweetener is also countered by its low water solubility . While in the first publications in 1971 and 1975 a 3,000-fold sweetening power compared to cane sugar was mentioned, this value could be checked after the total synthesis and corrected to the value 500-fold .
Polypodoside A
Polypodosid A is a closely related with osladin Sapoin that the rhizome of Polypodium glycyrrhiza (licorice fern , engl. Licorice remote occurs). It has a double bond at positions C7 and C8, but otherwise corresponds to osladin. Compared to a 6% sucrose solution, it is about 600 times sweeter, but like osladin, it is relatively insoluble in water. In addition, it has a light liquorice- like aftertaste and aftertaste.
Individual evidence
- ↑ a b c C.-R. Yang, O. Tanaka: Advances in Plant Glycosides, Chemistry and Biology. In: Proceedings of the International Symposium on Plant Glycosides, August 12-15, 1997, Kunming, China. Elsevier, 1999, ISBN 0-444-50180-0 .
- ↑ a b c L. O'Brien Nabors (Ed.): Alternative Sweeteners. 3rd, exp. Edition. Marcel Dekker, 2001, ISBN 0-8247-0437-1 , pp. 219f.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Science-Online-Lexica: Entry on Polypodium vulgare in the Lexicon of Medicinal Plants and Drugs. Retrieved August 26, 2008.
- ↑ H.-D. Belitz among other things: Textbook of food chemistry. 5th edition. Springer, Berlin et al. 2001, p. 431.
- ↑ J. Jizba et al.: The structure of osladin - the sweet principle of the rhizomes of polypodium vulgare L. In: Tetrahedron Lett. 18 (12), 1971, pp. 1329-1332, doi: 10.1016 / S0040-4039 (01) 96701-2 .
- ↑ M. Havel, V. Černý: Partial synthesis of osladine aglycone from solasodine. In: Collect Czech Chem Commun . 40, 1975, pp. 1579-1592, doi: 10.1135 / cccc19751579 .
- ↑ H. Yamada, M. Nishizawa: Total Synthesis of Intensely Sweet Saponin, Osladin. In: Synlett. 1, 1993, pp. 54-56.
- ^ A b H. Yamada, M. Nishizawa: Synthesis and Structure Revision of Intensely Sweet Saponin Osladin. In: J Org Chem. 60 (2), 1995, pp. 386-397, doi: 10.1021 / jo00107a018 .
- ↑ M. Nishizawa et al .: Structure Revision of Polypodoside A. Major Sweet Principle of Polypodium glycyrrhiza. In: Chem. Letters. 23 (8), 1994, pp. 1555-1558, doi: 10.1246 / cl.1994.1555 .

