Otto cycle
The Otto-cycle ( constant volume process ), the comparison process for the Otto engine , which according to the German inventor Nicholas Otto designated internal combustion engine . As a thermodynamic cycle it is clockwise, i.e. H. Heat energy is in kinetic energy ( work converted) ( heat engine ).
The term constant space is based on the assumption that the heat supply takes place with constant volume ( isochoric ). In contrast to this, there is the constant pressure process (also known as the diesel cycle ), in which the heat is supplied at constant pressure ( isobaric ). Both cycle processes are not suitable for calculating the thermodynamic conditions in piston engines. In practice, the mixed cycle process must be used.
At the beginning of the 20th century there were constant-space gas turbines that used the constant-space process with cyclical combustion of the gas mixture. These turbines, named after their designer Hans Holzwarth , did not need a compressor . They were displaced by the continuously operating gas turbines.
The comparison process
consists of four changes of state of an ideal gas within a closed system . So it does not involve any chemical conversion and also no charge exchange.
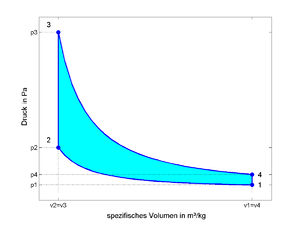
- 1 - 2: isentropic compression
- 2 - 3: isochoric heat supply (therefore constant space process!)
- 3 - 4: isentropic expansion
- 4 - 1: isochoric heat dissipation
The area enclosed by the line 1-2-3-4 in the diagrams corresponds to the specific process work .
Efficiency
For illustration and easy calculation of the state variables, an ideal gas with temperature-independent specific heat capacity is assumed as the working medium. The thermal efficiency of the ideal Otto process then does not depend on the amount of heat supplied and can be determined as follows:
The higher the compression ratio and the higher the isentropic exponent , the higher the efficiency.
- : Initial volume or expansion volume
- : Compression volume
- : Volume ratio (compression ratio)
- : Isentropic exponent
- : Specific heat capacity at constant pressure
- : Specific heat capacity at constant volume
The thermal efficiency of the constant space process is higher than that of the constant pressure process with the same compression ratio .
The equations for the state changes
The specific heat supply or heating energy determines the pressure or temperature increase. It does not play a role in terms of efficiency.
- ; Compaction pressure
- ( is the initial pressure, e.g. 1 bar)
- ; Compression temperature
- ( is the starting temperature before the compression stroke, e.g. 300 K)
- ; Temperature after the application of heat (maximum temperature)
- ( is the specific heat supplied)
- ; Pressure after the application of heat (maximum pressure)
- ; Pressure after expansion
The ideal gasoline engine
The ideal motor has no dissipation losses, mechanical friction losses, auxiliary units, cylinder cooling and leakage losses. The working gas has the same properties over the entire cycle and no flow losses. There is no mixing of the charge mixture with exhaust gas.
There are two- and four-stroke engines. A cycle consists of one piston stroke or half a crankshaft revolution. With the 4-stroke gasoline engine, the changes in state can be assigned to the work cycles as follows:
- 1st cycle = intake: the cylinder fills with fresh air 0 1.
- 2nd cycle = compression and heat supply: isentropic compression 1 2 and isochoric heat supply by igniting and burning the gas charge 2 3 at top dead center, i.e. with constant volume (constant space combustion ).
- 3rd cycle = work cycle: isentropic expansion 3 4.
- 4th cycle = blow-out cycle (heat dissipation): When the exhaust valve is opened, the exhaust gases expand outward 4 1 at bottom dead center without any further work , and the rest is pushed outward by the piston stroke 1 0. The heat contained in the exhaust gas is released into the environment. The ideal process does not take into account that the residual amount in the compression space does not reach the ambient state.
The real Otto engine
In a real gasoline engine, the knock resistance of the gas mixture limits the compression pressure. The changes in state of the constant space process do not correspond to the real engine, as time is required for combustion (see below). A significantly better approximation is obtained with a correspondingly adapted Seiliger cycle process . The air-gas mixture during compression and the fuel gases during expansion have different material properties and are strongly temperature-dependent (smaller isentropic exponent and greater heat capacity at high temperatures). Exhaust gases (burned air, mainly nitrogen, water vapor and carbon dioxide) have different thermodynamic properties than air-gas mixtures or fresh air . This is why the Seiliger process is too imprecise for realistic calculations. Compared to the comparison process, the real process in the engine also produces less work because:
- sucking in and pushing out is associated with friction losses (counter-clockwise loop between 0 and 1 in the pV diagram, gas exchange work )
- the combustion is not isochoric, but requires time in which the crankshaft continues to turn. Therefore, the ignition takes place before the top dead center, and the combustion is only completed after the o. T. The peak in the diagram at 3 is lower and rounded.
- Part of the energy supplied by the chemical reaction (in addition to incomplete combustion and endothermic formation of nitrogen oxide ) is lost without any work through heat transfer to the cylinder walls. The expansion curve is therefore below the ideal curve.
- the exhaust valve is opened before bottom dead center. The process area is rounded down at point 4.
The ratio of the work released in the engine to the theoretical work of the process is called the quality level. Real motors also have a mechanical power loss from friction and the required power for auxiliary and auxiliary drives (valves, pumps for oil and cooling water, fan), which can amount to approx. 10% of the nominal power and further reduce the efficiency.
literature
See also
- Equal pressure process or diesel process
- Seiliger process (mixed comparison process for piston engines)
- Carnot process (theoretical maximum thermodynamic efficiency)
- Joule process (constant pressure process in the turbine)
Web links
- Thermodynamics University of Munich (PDF; 9.7 MB)
- Uni Duisburg-Essen (PDF file; 2.50 MB)