Oxybutynin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Structural formula without specifying the stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Oxybutynin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| Melting point |
129-130 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Oxybutynin is an anticholinergic that is used for urinary incontinence and overactive bladder ; it is one of the advanced options for children wetting the bed .
It has an anticonvulsant effect on the smooth muscles of the bladder ( M. detrusor ) by competitively antagonizing the muscarinic acetylcholine receptor on the subtypes M1, M2 and M3 . As a calcium antagonist , it also has a direct spasmolytic effect on the smooth bladder muscles as well as a low local anesthetic component. By far the most common form of administration is in the form of a tablet, but a plaster ( trade name Kentera ) and an instillation set are also available on the German market .
As an anticholinergic, it also has an effect on excessive sweat production .
Stereochemistry
Oxybutynin contains a stereocenter . It is sold commercially as a racemate [1: 1 mixture of ( R ) -oxybutynine and ( S ) -oxybutynine]. The anticholinergic effect is mainly due to the ( R ) -enantiomer . In contrast, the calcium antagonism and the local anesthetic effect are not stereospecific.
| Enantiomers of oxybutynin | |
|---|---|
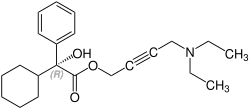 ( R ) -oxybutynine |
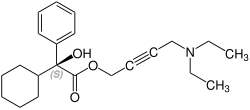 ( S ) -oxybutynine |
Clinical Effects
In two clinical trials involving patients with overactive bladder, oxybutynin transdermal patches with a release of 3.9 mg / day reduced incontinence episodes and increased mean urination significantly more than placebo. There was no difference between transdermally administered oxybutynin and delayed oral tolterodine administration.
Side effects
Typical anticholinergic side effects are dry mouth, constipation, blurred vision, fatigue, and dizziness. Anticholinergics can also cause delirium . This is dose-dependent and can be severe. The anticholinergic side effects are a common cause of drug withdrawal.
The active metabolite N -desethyloxybutynin is considered to be a major contributor to the undesirable effects. Its plasma level increases particularly sharply after the administration of a rapid-release oral dose (e.g. tablet). To counteract this, transdermal patches (Kentera ® ), prolonged-release tablets (Lyrinel OROS ® in Switzerland) and transdermal gels (Gelnique ® in the USA) have been developed. This also allows the frequency of application to be reduced from twice to once a day. The transdermal patch also bypasses the first-pass effect , so that the administered dose can be reduced. In the case of overflow incontinence due to diabetes or neurological diseases such as For example, multiple sclerosis or spinal cord injuries, oxybutynin can worsen overflow incontinence because the bladder contraction is caused by damage.
Clinical pharmacology
Oxybutynin is used medicinally as the hydrochloride - oxybutynin hydrochloride - and has a direct spasmolytic effect on the smooth muscles; it has an anticholinergic effect by inhibiting the acetylcholine effect on the smooth muscles.
Maximum plasma concentrations are reached after about 1 hour and the plasma half-life is 2–5 hours. The large inter-individual variation in blood values and the low urine concentration indicate that elimination occurs mainly via the liver.
Contraindications
Oxybutynin hydrochloride is contraindicated in untreated narrow-angle glaucoma because anticholinergics make the disease worse. Further contraindications are intestinal obstruction (also incomplete), hiatal hernia, reflux esophagitis, intestinal paralysis, megacolon , bladder emptying disorders and the like. a. Furthermore, it must not be used in cases of obstructive uropathy .
Web links
Individual evidence
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; P. 1199, ISBN 978-0-911910-00-1 .
- ↑ a b Ark Pharm: Oxybutynin , accessed October 11, 2018.
- ↑ Tupker RA, Harmsze AM, Deneer VH: Oxybutynin therapy for generalized hyperhidrosis. . In: Arch Dermatol . 142, No. 8, 2006, pp. 1065-1066. doi : 10.1001 / archderm.142.8.1065 . PMID 16924061 .
- ↑ Mijnhout GS, Kloosterman H, S Simsek, Strack van Schijndel RJ, Netelenbos JC .: Oxybutynin: dry days for patients with hyperhidrosis. . In: Neth J Med . 64, No. 9, 2006, pp. 326-328. PMID 17057269 .
- ↑ Schollhammer M, Misery L .: Treatment of hyperhidrosis with oxybutynin. . In: Arch Dermatol . 143, No. 4, 2007, pp. 544-545. doi : 10.1001 / archderm.143.4.544 . PMID 17438194 .
- ↑ Kachur JF, et al. R and S enantiomers of oxybutynin: pharmacological effects in guinea pig bladder and intestine , Journal of Pharmacology and Experimental Therapeutics , 247, pp. 867-872, 1988; PMID 2849672 .
- ↑ Noronha-Blob L, Kachur JF. Enantiomers of oxybutynin: in vitro pharmacological characterization at M 1 , M 2 and M 3 muscarinic receptors and in vivo effects on urinary bladder contraction, mydriasis and salivary secretion in guinea pigs , Journal of Pharmacology and Experimental Therapeutics , 256, pp. 562-567 , 1991; PMID 1993995 .
- ^ Mehta D (Ed.) 2006. British National Formulary 51. Pharmaceutical Press. ISBN 0-85369-668-3 .
- ^ Andreasen NC, Black DW, "Introductory Textbook of Psychiatry." American Psychiatric Publishing Inc. 2006.
- ↑ Allen B. Reitz, Suneel K. Gupta, Yifang Huang, Michael H. Parker, Richard R. Ryan: The preparation and human muscarinic receptor profiling of oxybutynin and N-desethyloxybutynin enantiomers . In: Med Chem . 3, No. 6, 2007, pp. 543-545. doi : 10.2174 / 157340607782360353 . PMID 18045203 .
- ^ Zobrist RH, et al. Pharmacokinetics of the R and S Enantiomers of Oxybutynin and N-Desethyloxybutynin Following Oral and Transdermal Administration of the Racemate in Healthy Volunteers , Pharmaceutical Research , 18, pp. 1029-1034, 2001; PMID 11496941 .
- ↑ Oki T, et al. Advantages for Transdermal over Oral Oxybutynin to Treat Overactive Bladder: Muscarinic Receptor Binding, Plasma Drug Concentration, and Salivary Secretion , Journal of Pharmacology and Experimental Therapeutics , 316, pp. 1137-1145, 2006; PMID 16282521 .
- ↑ drugs.com: Oxybutynin . Retrieved August 30, 2012.
- ↑ a b J. Douchamps, F. Derenne, A. Stockis, D. Gangji, M. Juvent, A. Herchuelz: The pharmacokinetics of oxybutynin in it. In: European Journal of Clinical Pharmacology . Volume 35, Number 5, 1988, pp. 515-520, PMID 3234461 .
- ↑ americareoncall.com: Oxybutynin ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. . Retrieved August 30, 2012.