Tolterodine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
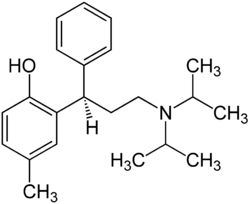
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Tolterodine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| Brief description |
Crystals [Tolterodine · ( R , R ) -Tartrate] |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class |
Urinary tract antispasmodics |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | |||||||||||||||||||
| solubility |
soluble in methanol, slightly soluble in ethanol, practically insoluble in toluene. Solubility in water: 12 mg ml −1 [tolterodine ( R , R ) -tartrate] |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data |
10–20 mg kg −1 ( LD 50 , mouse , iv , tolterodine ( R , R ) -tartrate) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tolterodine (trade name Detrusitol ) is a chiral drug that is used as a specific and competitive antagonist at muscarinic receptors in patients with overactive bladder syndrome as a urological agent.
Clinical information
Application areas (indications)
The active ingredient is used for symptomatic therapy in patients with an increased urinary frequency (frequent urination), an imperative, increased urge to urinate (sudden urge to urinate), urge incontinence (sudden loss of control over urination), as can occur in overactive bladder syndrome .
Contraindications (contraindications)
- If you are hypersensitive to tolterodine
- Urinary retention
- Stomach retention (when the stomach does not empty properly)
- Inadequately treated or untreated narrow-angle glaucoma (increased pressure in the eye even with appropriate treatment),
- Myasthenia gravis (a neurological disease that causes muscle weakness)
- Severe ulcerative colitis (severe inflammation of the colon causing ulceration and bleeding)
- Toxic megacolon (a very serious complication of colitis)
Adverse effects (side effects)
The side effects include anticholinergic effects.
Other Information
Chemical and pharmaceutical information
Tolterodine tartrate is a white, crystalline powder. The pKa value is 9.87 and the solubility in water is 12 grams per liter. It is soluble in methanol , slightly soluble in ethanol , and practically insoluble in toluene . The octanol-water partition coefficient (Log D) is 1.83 at a pH of 7.3.
literature
- Eckhard Petri : Gynecological urology: Aspects of interdisciplinary diagnostics and therapy . Thieme, Stuttgart 2001, ISBN 978-3-13-639103-7 .
- Berdel V. et al. (Ed.); Diehl Classen: Internal medicine with StudentConsult access . Urban & Fischer in Elsevier, Munich 2006, ISBN 978-3-437-44405-0
Web links
- Information at MedlinePlus
- FDA label for Detrol ® (tolterodine tartrate) - Status: April 2010 (PDF; 148 kB) on the website of the Food and Drug Administration FDA
Individual evidence
- ↑ a b c The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition, 2006, p. 1637, ISBN 978-0-911910-00-1 .
- ↑ a b Datasheet Tolterodine L-tartrate from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ a b Information for professionals (summary of the product characteristics) Detrusitol ® from PFIZER PHARMA GmbH - as of April 2008 .
- ↑ Torsten Kratz, Albert Diefenbacher: Psychopharmacotherapy in old age. Avoidance of drug interactions and polypharmacy. In: Deutsches Ärzteblatt. Volume 116, Issue 29 f. (July 22) 2019, pp. 508-517, p. 511.
- ↑ FDA label for Detrol ® (tolterodine tartrate) - as of 2012 .