PRO-LAD
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
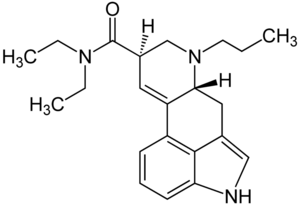
|
||||||||||
| General | ||||||||||
| Surname | PRO-LAD | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 22 H 29 N 3 O | |||||||||
| Brief description |
colorless solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| Mechanism of action | ||||||||||
| properties | ||||||||||
| Molar mass | 351.49 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
87-88 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
PRO-LAD (engl. N - Pro pyl - normal l ysergic a cid d iethylamide ) is a chemically prepared derivative of lysergic acid , which in ergot alkaloids naturally occurs. Even in very small doses , it produces long-lasting hallucinogenic effects. Pharmacologically, PRO-LAD belongs to the group of serotonin-related psychedelic substances.
chemistry
Chemically, PRO-LAD belongs to the Ergoline structural class . The tetracyclic ergoline is characteristic of the chemical structure of ergot alkaloids . The ergolines found in nature are methylated on the nitrogen in position 6 of the ergoline system . In contrast to LSD, PRO-LAD has an N 6 - propyl group instead of an N 6 - methyl group .
Effect on humans
The duration of an uncomplicated PRO-LAD experience is usually between 6 and 8 hours, depending on the dosage, body weight and age. The effective dose is given as 100–200 µg . PRO-LAD acts on the receptor subtypes of the serotonin (5-HT 2 ) receptors and the dopamine receptors .
literature
- Alexander Shulgin , Ann Shulgin: TiHKAL, the Continuation . Transform Press, Berkeley 1997, ISBN 0-9630096-9-9 ( online ).
- Andrew J. Hoffman, David E. Nichols: Synthesis and LSD-like discriminative stimulus properties in a series of N (6) -alkyl norlysergic acid N, N-diethylamide derivatives . In: Journal of Medicinal Chemistry . tape 28 , no. September 9 , 1985, ISSN 0022-2623 , pp. 1252-1255 , doi : 10.1021 / jm00147a022 .
- T. Niwaguchi, Y. Nakahara, H. Ishii: Studies on lysergic acid diethylamide and related compounds. IV. Syntheses of various amide derivatives of norlysergic acid and related compounds. In: Yakugaku Zasshi. Volume 96, Number 5, May 1976, pp. 673-678, ISSN 0031-6903 . PMID 987200 .
- Robert C. Pfaff, Xuemei Huang, Danuta Marona-Lewicka, Robert Oberlender and David E. Nichols: Lysergamides Revisited. In: NIDA Research Monograph 146: Hallucinogens: An Update. P. 52, 1994, United States Department of Health and Human Services.
See also
Web links
Individual evidence
- ↑ a b c Alexander and Ann Shulgin: PRO-LAD . In: TiHKAL. Transform Press, Berkeley 1997, ISBN 0-9630096-9-9
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Robert C. Pfaff, Xuemei Huang, Danuta Marona-Lewicka, Robert Oberlender and David E. Nichols: Lysergamides Revisited. ( Memento of the original from July 23, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. In: NIDA Research Monograph 146: Hallucinogens: An Update. P. 52, 1994, United States Department of Health and Human Services.


