Pantolactone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
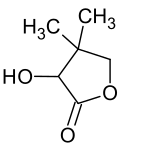
|
|||||||||||||||||||
| Structural formula of pantolactone without information on stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Pantolactone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 10 O 3 | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 130.14 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.25 g cm −3 |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| boiling point |
120–122 ° C (20 hPa, R -form) |
||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Pantolactone is a chemical compound from the group of substituted lactones .
Isomers
Pantolactone contains a stereocenter , so it is chiral and occurs in two enantiomeric forms , ( R ) -pantolactone and ( S ) -pantolactone. Racemic pantolactone [synonym: ( RS ) -pantolactone] is a 1: 1 mixture of the ( R ) - and the ( S ) -enantiomer.
| Isomers of pantolactone | ||
| Surname | ( S ) -pantolactone | ( R ) -pantolactone |
| other names | (+) - pantolactone | (-) - pantolactone |
| Structural formula |

|

|
| CAS number | 5405-40-3 | 599-04-2 |
| 79-50-5 (racemate) | ||
| EC number | 626-470-2 | 209-963-3 |
| 201-210-7 (racemate) | ||
| ECHA info card | 100.149.096 | 100.009.059 |
| 100.001.101 (racemate) | ||
| PubChem | 736053 | 439368 |
| 989 (racemate) | ||
| Wikidata | Q76962655 | Q27102042 |
| Q22829045 (racemate) | ||
Occurrence
Pantolactone is found in wine and sherry flavor.
Extraction and presentation
( R ) -Pantolactone is obtained by degrading pantothenic acid or by enantioselective reduction of the corresponding oxolactone . Both stereoisomers can be isolated separately by resolution with ( R ) - and ( S ) - phenylethanamine .
properties
Pantolactone is a colorless solid that is soluble in water.
use
In addition to being used as a chiral auxiliary for diastereoselective Diels-Alder reactions , ( R ) -pantolactone is used as a synthetic building block from the so-called “chiral pool”, for example for the synthesis of bryostatin and the antibiotic elfamycin . It is also used as a humectant in the cosmetics industry.
Individual evidence
- ↑ a b c d e f g data sheet DL-α-Hydroxy-β, β-dimethyl-γ-butyrolactone, purum, ≥97.0% (T) from Sigma-Aldrich , accessed on October 19, 2019 ( PDF ).
- ↑ a b c d e f Entry on pantolactone. In: Römpp Online . Georg Thieme Verlag, accessed on October 19, 2019.
- ↑ Joachim Paust, Sigberg Pfohl, Werner Reif, Wolfram Schmidt: Racemate resolution of pantolactone with new chiral amines. In: Justus Liebig's Annals of Chemistry. 1978, 1978, p. 1024, doi : 10.1002 / jlac.197819780612 .
- ↑ Paul Präve: Handbook of biotechnology . Oldenbourg Industrieverlag, 1994, ISBN 978-3-8356-6223-0 , p. 719 ( limited preview in Google Book search).
- ↑ dr-pfleger.de: INCI-Lexikon - Service for Patients - Products - dr-pfleger.de , accessed on October 19, 2019