Parecoxib
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
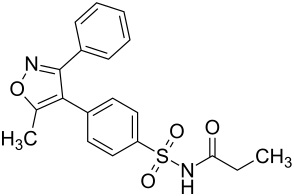
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Parecoxib | |||||||||||||||||||||
| other names |
N - {[4- (5-methyl-3-phenylisoxazol-4-yl) phenyl] sulfonyl} propionamide ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 19 H 18 N 2 O 4 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
selective inhibition of COX-2 |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 370.42 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Parecoxib is a drug from the group of selective COX-2 inhibitors (cyclooxygenase-2 inhibitors) and is marketed by Pfizer under the trade name Dynastat . Parecoxib has not been approved in the USA due to safety concerns and has been withdrawn from the market in Switzerland. The sodium salt is used.
pharmacology
Pharmacokinetics
Parecoxib is the water-soluble and parenterally administrable prodrug of the orally administered valdecoxib (trade name: Bextra ), which was formerly marketed but discontinued . Activation takes place via enzymatic cleavage with a half-life of 22 minutes to form valdecoxib, the half-life of which as the actual active substance is approx. 8 hours, with elimination via the liver.
indication
It is approved for short-term postoperative pain therapy, preferably after dental, orthopedic and gynecological operations. Little experience is available for gastrointestinal or urological interventions. When using the device, the individual patient risk with regard to cardiovascular diseases must be weighed up.
Mechanism of action
The analgesic effect is achieved through a selective inhibition of the enzyme cyclooxygenase-2 (COX-2), with an effect occurring after approx. 7-13 minutes and the effect corresponding to an effect of 12 mg morphine , which saves opioids or opioid therapy can be avoided. It should also be noted that the substance has no effect on platelet aggregation and thus on bleeding time.
Side effects
The following side effects should be considered during treatment (selection):
- increased creatinine in the blood (kidney damage)
- gastroduodenal ulcer
- Hypokalemia
- peripheral edema
- postoperative anemia
- insomnia
- Worsening of existing hypertension
- severe cardiovascular complications
Contraindications
The following are absolute contraindications (selection):
- Allergy to acetylsalicylic acid , NSAIDs or sulfonamides
- inflammatory bowel disease
- third trimester of pregnancy and lactation
- Heart failure (NYHA II-IV)
- peptic ulceration or gastrointestinal bleeding
- severe liver problems
- Clinically proven coronary artery disease, peripheral arterial disease and / or cerebrovascular disease
application
Parecoxib can be administered im or iv .
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Parecoxib
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of N - {[4- (5-methyl-3-phenyl-1,2-oxazol-4-yl) phenyl] sulfonyl} propanamide in the Classification and Labeling Inventory of the European Chemicals Agency is shown, which is derived from a self-classification by the distributor ( ECHA), accessed on July 04, 2020.
- ↑ Entry on parecoxib. In: Römpp Online . Georg Thieme Verlag, accessed on July 21, 2019.
- ↑ Mutschler, Geisslinger, Kroemer, Ruth, Schäfer-Korting, Mutschler drug effects, 9th edition, 2008, ISBN 3-8047-1952-X .
- ↑ a b Technical information on Dynastat.
- ↑ a b c d ABDA database (as of August 16, 2008) of DIMDI.
- ↑ a b c Information page of the manufacturer about Dynastat .
- ↑ Nussmeier NA, Whelton AA, Brown MT, et al. : Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery . In: N. Engl. J. Med. . 352, No. 11, March 2005, pp. 1081-91. doi : 10.1056 / NEJMoa050330 . PMID 15713945 .